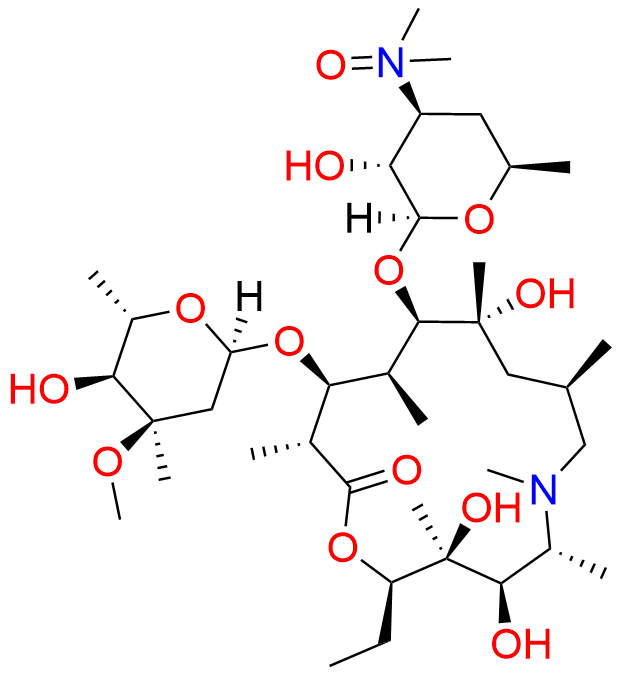
Azithromycin EP impurity L
| CAT. No. | CP-A37012 |
|---|---|
| CAS. No. | 90503-06-3 |
| Mol. F. | C38H72N2O13 |
| Mol. Wt. | 764.98 |
| Stock Status | In Stock |
Product Description
- Category:Impurity Standards
- Synonyms:Azithromycin 3'-N-Oxide ; Azithromycin N-Oxide (USP)
- Chemical Name:(2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethyloxidoamino)-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one