Manufacturer of Pharmaceutical Standards
Serving to 500+ Customers in 35+ Countries
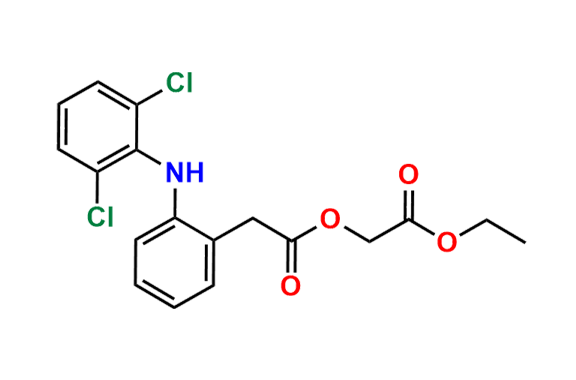
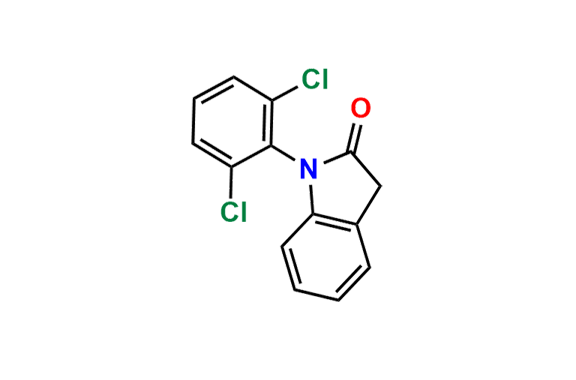
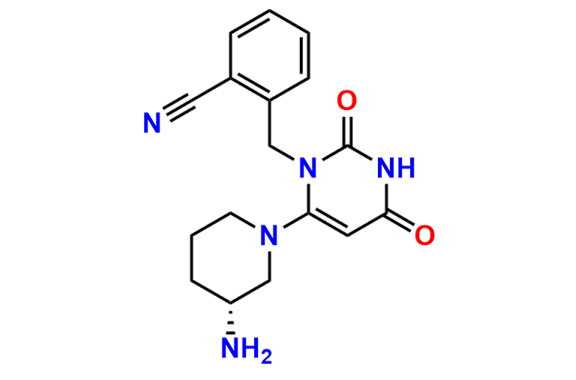
Are you in search of Nitrosamine impurities?
We have over 1500 genotoxic nitrosamine impurities available with us.
View Compounds


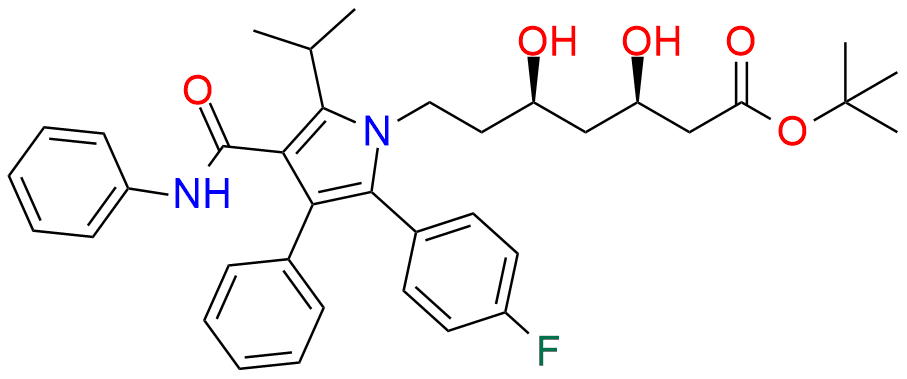
GATIFLOXACIN
Gatifloxacin works by inhibiting bacterial DNA gyrase and topoisomerase IV enzymes which are essential for the replication and maintenance of bacterial DNA
View Compounds