
A Detailed Overview of Rosuvastatin Impurities: EP, USP, and Global Guidelines
At Chemicea Pharmaceutical, we specialize in delivering high-quality pharmaceutical reference standards, including a comprehensive range of Rosuvastatin impurities. These impurities are synthesized and characterized in compliance with international pharmacopeial guidelines such as the European Pharmacopoeia (EP), United States Pharmacopeia (USP), and other global standards.
Rosuvastatin, a widely prescribed statin, plays a crucial role in managing cholesterol levels and reducing cardiovascular risks. Ensuring the purity, stability, and efficacy of Rosuvastatin is essential, and the ability to identify and manage impurities is a key factor in maintaining product quality and regulatory compliance.
What Are Rosuvastatin Impurities?
Rosuvastatin impurities refer to substances that may form during the manufacturing, storage, or degradation of the drug. These impurities can affect the drug’s purity and efficacy. They must be identified, controlled, and minimized to meet pharmacopeial standards
How Rosuvastatin Impurities Impact Pharmaceutical Quality
Impurities in Rosuvastatin can arise from several sources, such as synthesis by-products, degradation products due to environmental factors, or process inconsistencies. Proper identification and management of these impurities are vital for maintaining the drug’s quality, safety, and efficacy, ensuring compliance with EP, USP, and other regulatory standards.
Rosuvastatin Structure
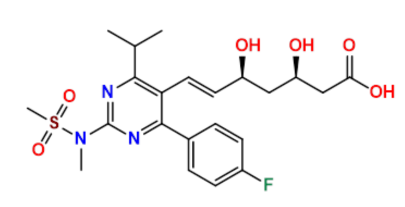
Rosuvastatin Chemical Details
- CAS Number: 287714-41-4
- Chemical Formula: C22H28FN3O6S
- Molecular Weight: 481.54 g/mol
- IUPAC Name: (3R,5S,E)-7-(4-(4-fluorophenyl)-6-isopropyl-2-(N-methylmethylsulfonamido)pyrimidin-5-yl)-3,5-dihydroxyhept-6-enoic acid
- Synonyms:
- Crestor
- Zuvamor
- Rosuless
These chemical details form the foundation for understanding Rosuvastatin's synthesis, stability, and potential impurities, ensuring compliance with pharmacopeial standards.
The Chemistry of Rosuvastatin
Rosuvastatin is an HMG-CoA reductase inhibitor that reduces cholesterol levels in the body. Its chemical structure features a fluorophenyl group and a pyrimidine ring, making it highly effective in inhibiting cholesterol production. However, this complex structure also presents challenges in managing potential impurities during the drug's synthesis and storage.
Understanding the chemistry of Rosuvastatin is key to identifying impurities and ensuring that the drug meets global regulatory guidelines.
Pharmaceutical Applications of Rosuvastatin
- Primary Use: Rosuvastatin is used to lower cholesterol and triglyceride levels, which helps prevent cardiovascular diseases such as heart attacks and strokes.
- Off-Label Uses: It has also been explored for its potential to reduce inflammation and treat lipid disorders.
The purity and stability of Rosuvastatin are vital to its success in these applications. Controlling impurities ensures that Rosuvastatin remains safe, effective, and compliant with global pharmacopeial standards.
Rosuvastatin and Its Impurities Tree Diagram
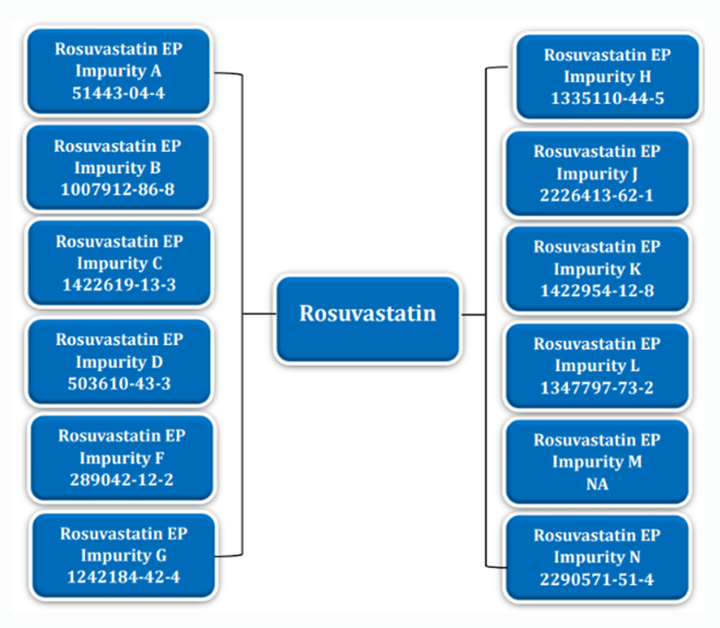
Understanding Rosuvastatin Impurities
Impurities in Rosuvastatin can be classified into three main categories:
- Degradation Impurities: Formed when Rosuvastatin is exposed to heat, light, or pH changes during storage.
- Process Impurities: By-products that result from incomplete reactions or side reactions during synthesis.
- Potential Impurities: Hypothetical impurities predicted based on the synthetic pathway and degradation mechanisms.
Managing these impurities is essential for maintaining the stability and efficacy of the drug. At Chemicea Pharmaceutical, we offer high-quality reference standards to help pharmaceutical companies ensure compliance with EP, USP, and global guidelines.
Key Rosuvastatin Impurities Offered by Chemicea
At Chemicea Pharmaceutical, we provide an extensive range of Rosuvastatin impurities. These impurities are critical for quality control, stability testing, and method development in pharmaceutical research. Below is a list of key Rosuvastatin impurities, arranged alphabetically:
|
CAT No. |
Impurity Name |
CAS Number |
|
NA |
||
These impurities are monitored and controlled to ensure product consistency and meet stringent regulatory standards.
Classification and Description of Rosuvastatin Impurities
Rosuvastatin EP Impurity A (CAS No. 851443-04-4)
- Type: Process Impurity
- Description: A by-product of the synthesis process, typically formed due to incomplete reactions.
- Role: Important in process control to ensure efficient and consistent production.
Rosuvastatin EP Impurity B (CAS No. 1007912-86-8)
- Type: Degradation Impurity
- Description: Forms when Rosuvastatin is exposed to environmental factors like light and heat, causing degradation.
- Role: Used in stability testing to ensure product efficacy over time.
Rosuvastatin EP Impurity C (CAS No. 1422619-13-3)
- Type: Potential Impurity
- Description: A predicted impurity that may form under specific synthetic conditions.
- Role: Important in method development for impurity profiling.
Rosuvastatin EP Impurity D (CAS No. 503610-43-3)
- Type: Process Impurity
- Description: A by-product that may form due to variations in reaction temperatures or reagent quality.
- Role: Monitored for process optimization and batch consistency.
Rosuvastatin EP Impurity F (CAS No. 289042-12-2)
- Type: Degradation Impurity
- Description: Forms under oxidative conditions, such as prolonged exposure to air or light.
- Role: Used in stability testing to assess oxidative degradation and shelf life.
Rosuvastatin EP Impurity G (CAS No. 1242184-42-4)
- Type: Process Impurity
- Description: A by-product of synthesis, typically occurring under non-ideal conditions.
- Role: Monitored to ensure product purity and minimize impurities during manufacturing.
Rosuvastatin EP Impurity H (CAS No. 1335110-44-5)
- Type: Process Impurity
- Description: By-product of incomplete synthesis reactions or impurities in reagents.
- Role: Ensures product consistency and adherence to pharmacopeial standards.
Rosuvastatin EP Impurity J (CAS No. 2226413-62-1)
- Type: Potential Impurity
- Description: Hypothetical impurity based on specific reaction pathways during synthesis.
- Role: Monitored for comprehensive impurity profiling and quality control.
Rosuvastatin EP Impurity K (CAS No. 1422954-12-8)
- Type: Process Impurity
- Description: Formed as a by-product during synthesis under sub-optimal conditions.
- Role: Important for maintaining process consistency and minimizing impurities.
Rosuvastatin EP Impurity L (CAS No. 1347797-73-2)
- Type: Process Impurity
- Description: Side product formed under non-ideal synthesis conditions.
- Role: Monitored for process control and product quality consistency.
Rosuvastatin EP Impurity M (CAS No. NA)
- Type: Degradation Impurity
- Description: Forms due to environmental stress, such as heat or light exposure.
- Role: Used in stability testing to ensure product longevity.
Rosuvastatin EP Impurity N (CAS No. 2290571-51-4)
- Type: Potential Impurity
- Description: Hypothetical by-product predicted under certain synthetic conditions.
- Role: Important for method development and ensuring impurity control during manufacturing.
How Chemicea Helps Ensure Quality
At Chemicea Pharmaceutical, we provide certified reference standards for Rosuvastatin impurities, helping pharmaceutical companies maintain regulatory compliance and meet the highest quality standards. By utilizing our expertise in impurity profiling and synthesis, we ensure that your products are safe, effective, and fully compliant with international standards.
Conclusion
Managing Rosuvastatin impurities is crucial for maintaining the quality, safety, and efficacy of pharmaceutical products. Chemicea Pharmaceutical provides the expertise and certified reference standards needed to support your quality control and regulatory compliance efforts.
For more information on our Rosuvastatin impurities or to explore our comprehensive product catalog, contact our team today. We are committed to supporting your pharmaceutical development and ensuring your products meet the highest industry standards.