
Why N-nitroso Amlodipine is not feasible?
Amlodipine
CAT No-CP-A5000 | CAS NO.-88150-42-9
Amlodipine was patented in 1982, and in 1990 it was approved for medicinal use.It is available as a generic medication. Amlodipine is a calcium channel blocker. Chemically described as 3-ethyl 5-methyl 2-oxo-4-(2-propenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylat with cas no 88150-42-9.Its molecular formula is C20H25ClN2O5, with a molecular weight of 408.88 g/mol.
Amlodipine is a medication primarily used to treat high blood pressure (hypertension) and chest pain (angina). Amlodipine works by relaxing and widening the blood vessels, allowing blood to flow more easily and reducing the workload on the heart.
Mechanism:
Amlodipine is a calcium channel blocker. Calcium channel blockers are medicines used to lower blood pressure. Calcium channel blockers (CCBs) work by blocking specific channels that allow calcium to enter cells in the walls of blood vessels and the heart. When these channels are blocked, the amount of calcium entering the cells decreases. As a result, the blood vessels relax, leading to a widening of the vessels, and the heart muscle receives more oxygenated blood. This mechanism helps to lower blood pressure and alleviate symptoms of angina by improving blood flow to the heart. In essence, CCBs promote relaxation of blood vessels and enhance blood supply to the heart muscle, contributing to their effectiveness in treating hypertension and angina.By blocking calcium channels in smooth muscle cells, amlodipine reduces peripheral vascular resistance, resulting in decreased blood pressure. It's often prescribed alone or in combination with other antihypertensive medications to achieve optimal blood pressure control.
Side Effects:
• Edema (swelling) in the ankles or feet
• sensations of dizziness
• Flushing of the skin
• Fatigue
• Headaches
• Abdominal discomfort
The recent curiosity surrounding the synthesis of N-nitroso Amlodipine stems from growing interest in various N-nitroso compounds, which are often discussed in the context of their potential biological activities or health implications. However, the specific synthesis of N-nitroso Amlodipine poses significant challenges, making it a subject of speculative or theoretical interest rather than practical application.
Why N-nitroso Amlodipine is not feasible?
N-nitroso compounds are typically synthesized by reacting secondary amines with nitrosating agents, however, Any compound containing the secondary amine functional group is expected to react with nitrosating agents to produce nitrosamine. These molecules are of concern because nitrosamine impurities are probable human carcinogens
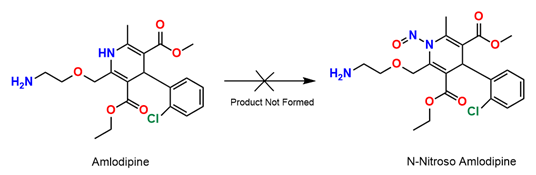
The synthesis of N-nitroso Amlodipine presents significant challenges, which are largely attributable to the unique structural features of Amlodipine itself. A key factor complicating its potential for N-nitrosation is the incorporation of its nitrogen atom into an aromatic system. In the structure of Amlodipine, the nitrogen's lone pair electrons are delocalized as part of an aromatic ring, which fundamentally alters its chemical reactivity and basicity compared to conventional secondary amines that are typically targeted for N-nitrosation.