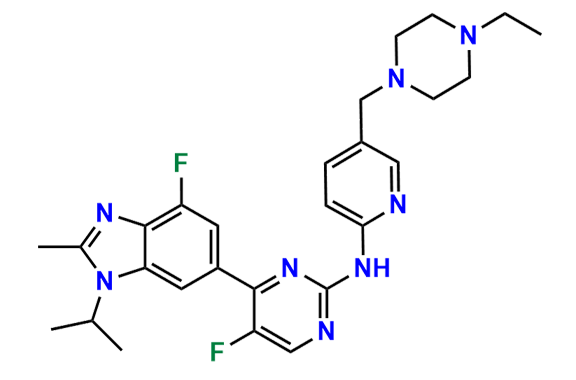
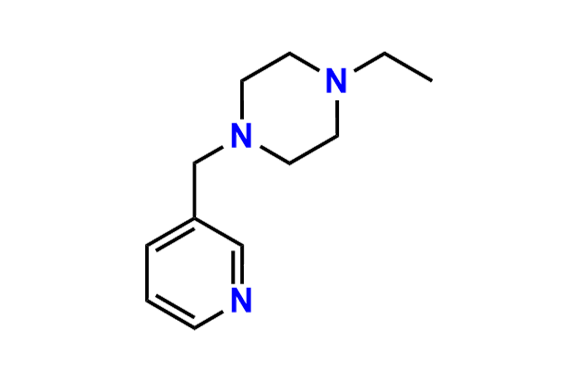
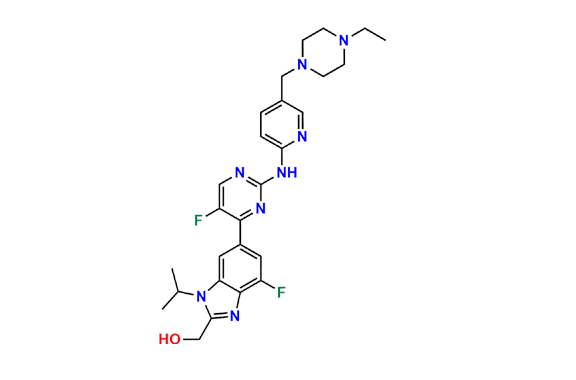
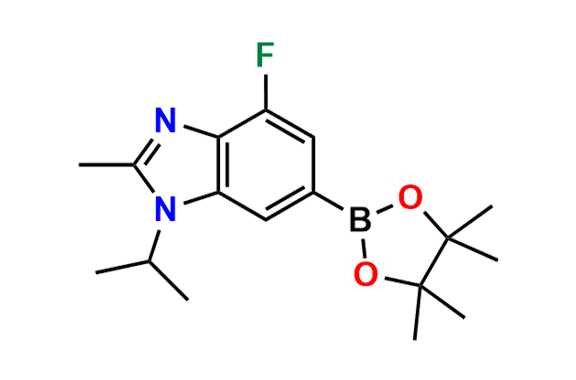
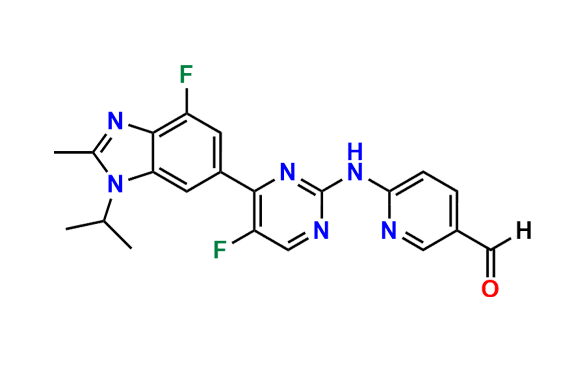
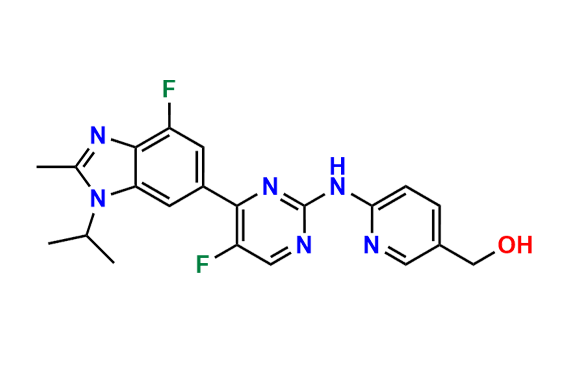
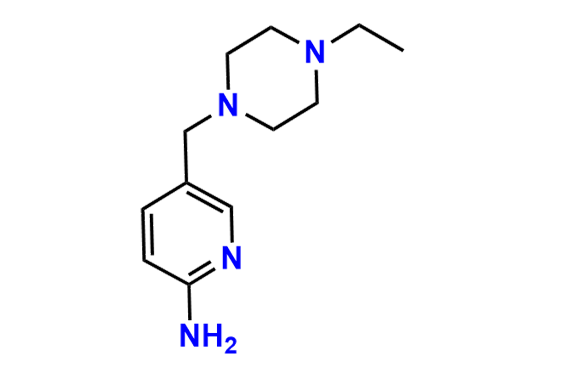
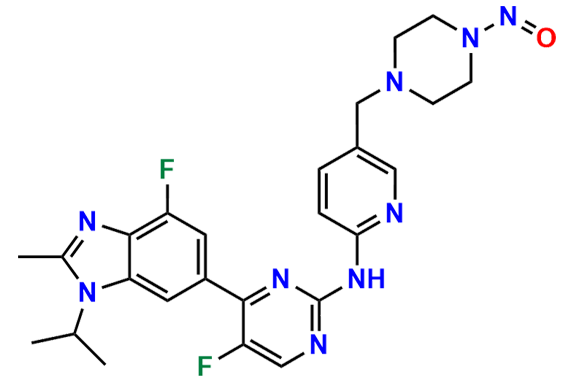
Abemaciclib
| CAT. No. | CP-A97000 |
|---|---|
| CAS. No. | 1231929-97-7 |
| Mol. F. | C27H32F2N8 |
| Mol. Wt. | 506.61 |
| Stock Status | Custom Synthesis |
| Rel. Cas No | 2089320-70-5 (HCl salt) ; 1231930-82-7 (Methanesulfonic acid) |
- Synonyms: NA
- Chemical Name: N-(5-((4-Ethylpiperazin-1-yl)methyl)pyridin-2-yl)-5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)pyrimidin-2-amine