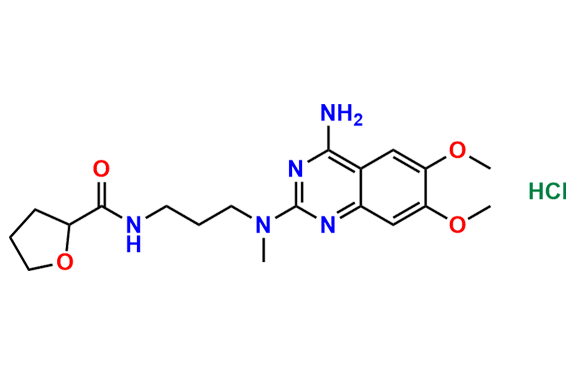
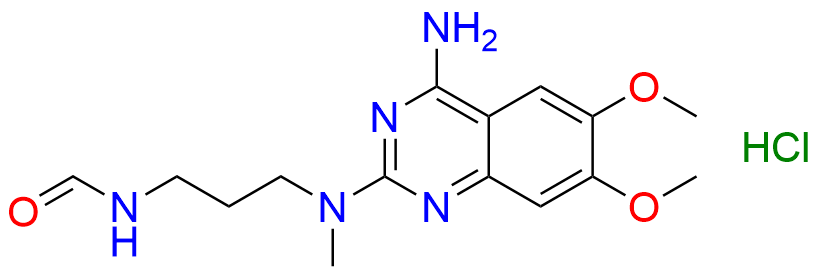
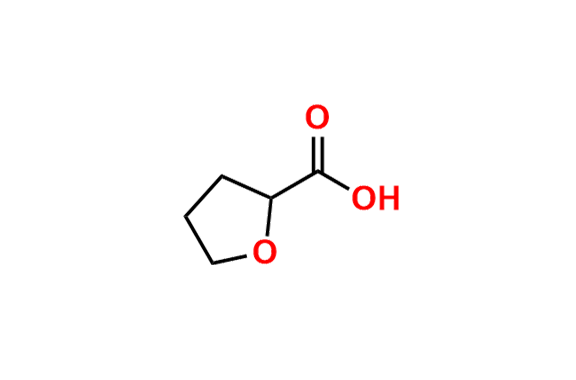
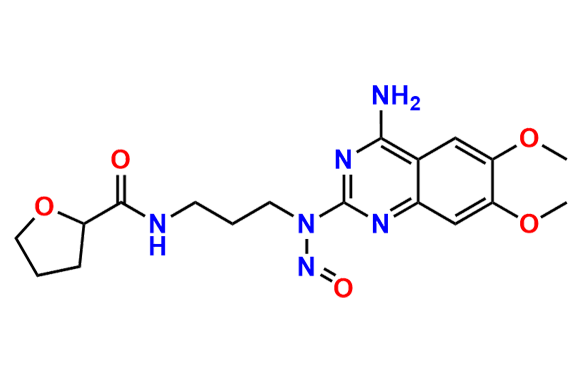
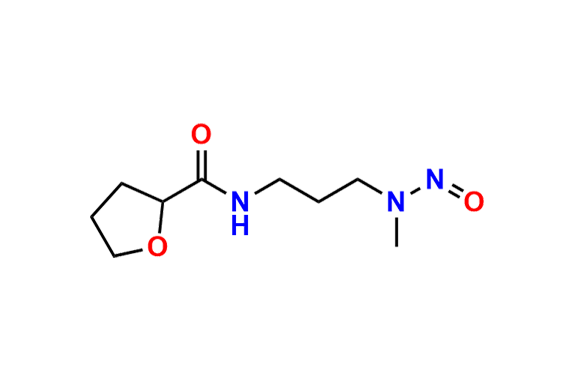
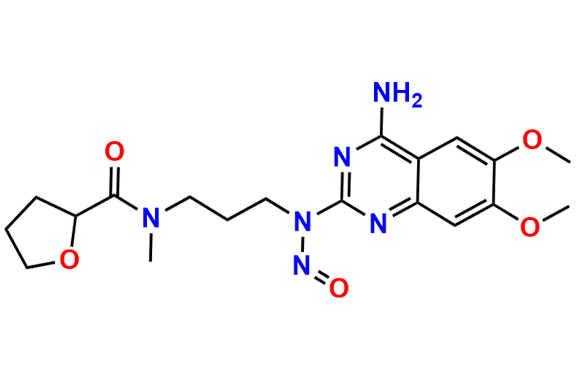
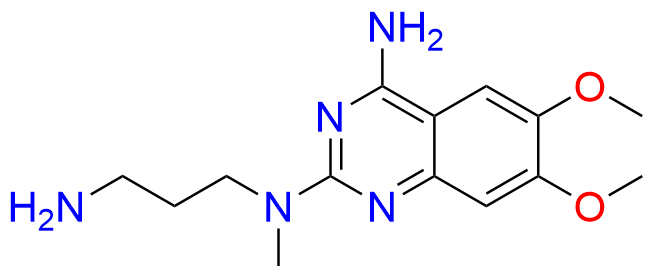
Alfuzosin EP Impurity D
| CAT. No. | CP-A71004 |
|---|---|
| CAS. No. | 76362-29-3 |
| Mol. F. | C14H21N5O2 |
| Mol. Wt. | 291.35 |
| Stock Status | In Stock |
| Rel. Cas No | 81403-69-2(HCl Salt) |
- Category: Impurity Standards
- Synonyms: Alfuzosin Aminopropyl Impurity ; Deacylated alfuzosin
- Chemical Name: N2-(3-Aminopropyl)-6,7-dimethoxy-N2-methylquinazolin-2,4-diamine (as per EP )

 VIEW COA
VIEW COA