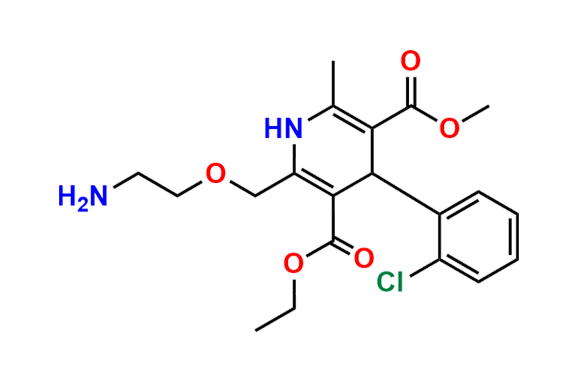
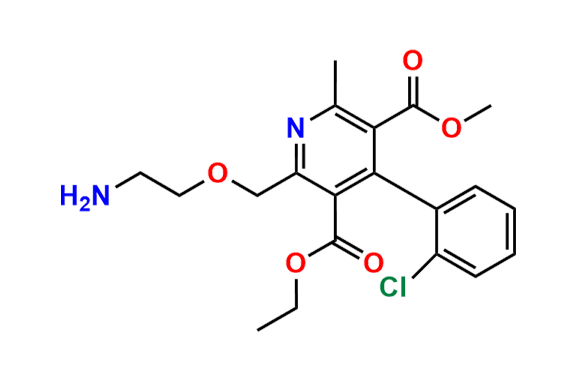
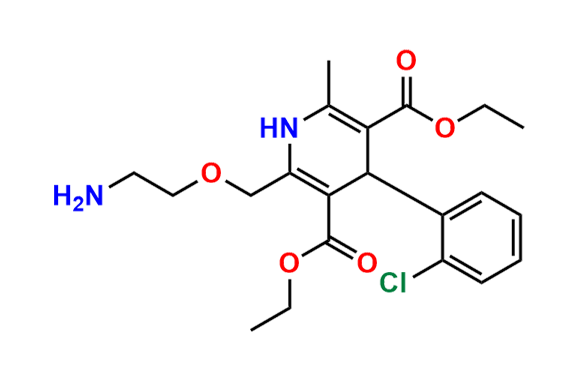
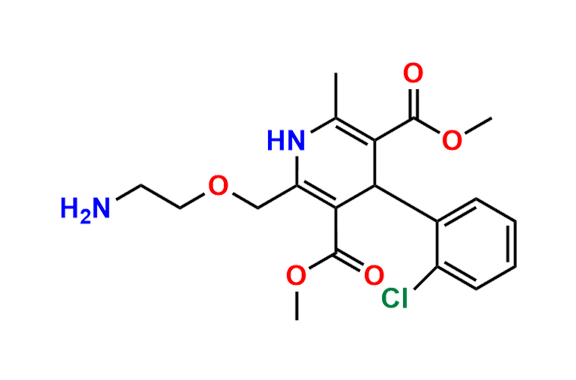
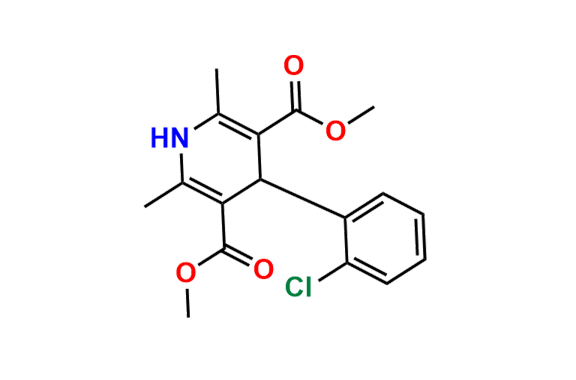
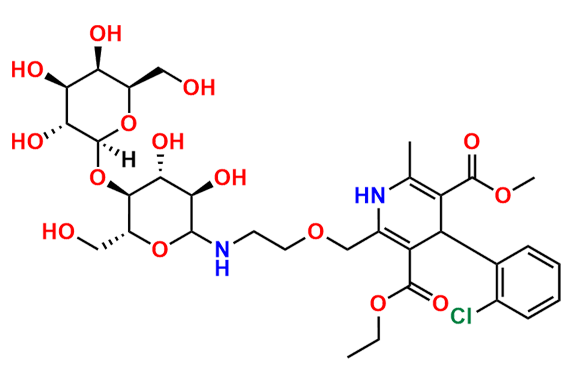
Amlodipine N-Lactoside impurity
| CAT. No. | CP-A5015 |
|---|---|
| CAS. No. | 2173291-00-2 |
| Mol. F. | C32H45ClN2O15 |
| Mol. Wt. | 733.16 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: Amlodipine Lactose Adduct (Mixture of Diastereomers)
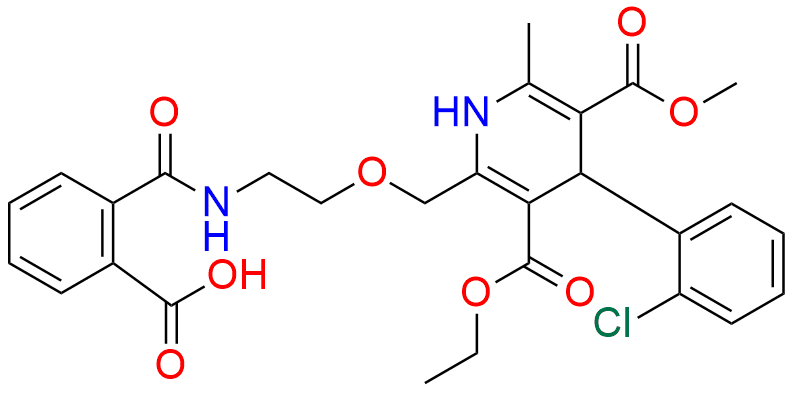
- Chemical Name: 3-ethyl 5-methyl 4-(2-chlorophenyl)-2-((2-(((3R,4R,5S,6R)-3,4-dihydroxy-6-(hydroxymethyl)-5-(((2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)amino)ethoxy)methyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
FAQ
It is a specified impurity formed when Amlodipin interacts with lactose, commonly occurring during storage, used in pharmaceutical quality control.
It is monitored to ensure quality and safety in Amlodipin formulations, meeting regulatory guidelines.
It forms as a reaction product between Amlodipin and lactose, typically under certain storage conditions.
Formation can be managed by controlling storage conditions and implementing routine testing.
Detection and quantification of Amlodipin Lactose Adduct use advanced analytical methods like GC-MS, LC-MS, and HPLC-MS.
Chemicea provides COA, H-NMR, MASS, HPLC, and TGA reports as standard, with additional documents available upon request.
Yes, Chemicea\'s documentation meets standards required by major regulatory bodies like the FDA, EMA, and other authorities.
Amlodipin Lactose Adduct is stable for shipping at room temperature, with specific storage guidelines provided in the COA.
Yes, both standard and customized pack sizes are available based on client requirements.
Chemicea provides Amlodipin Lactose Adduct as a reference standard, supporting impurity analysis, validation, and regulatory compliance.

 VIEW COA
VIEW COA