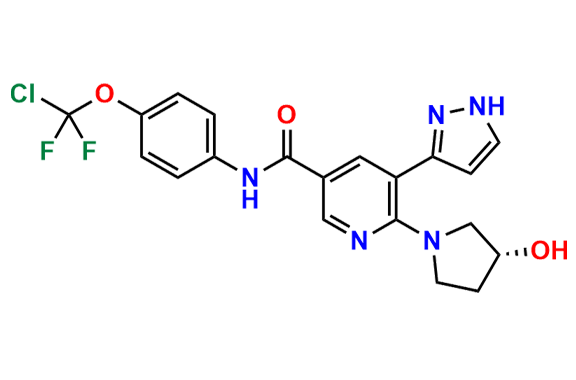
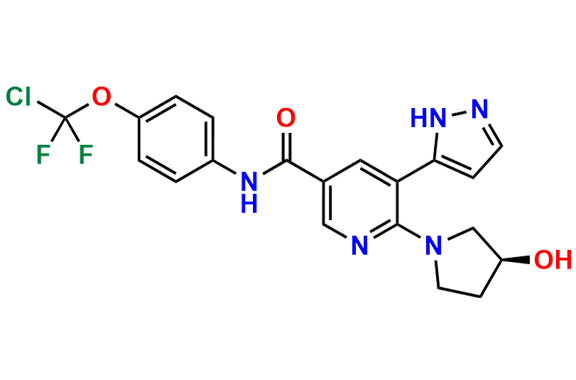
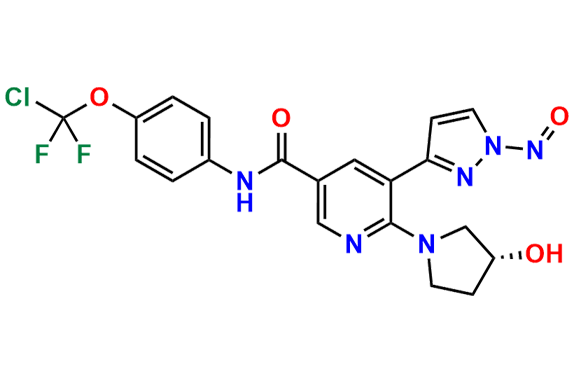
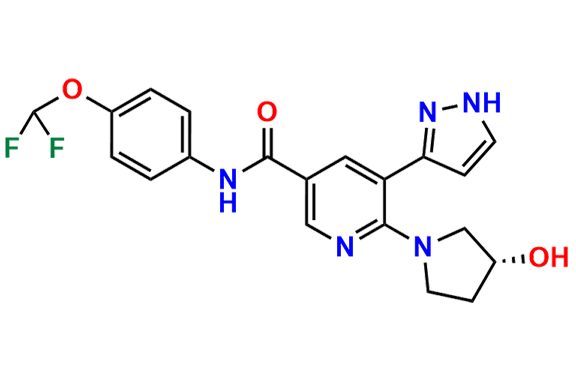
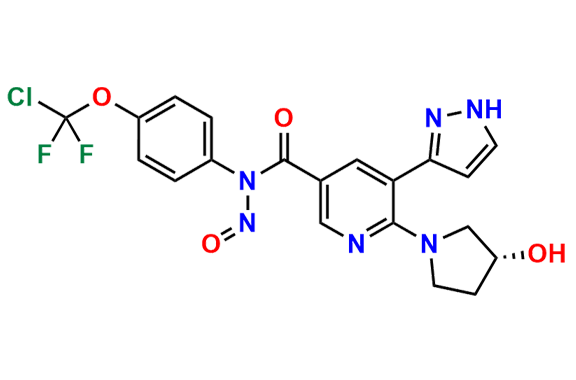
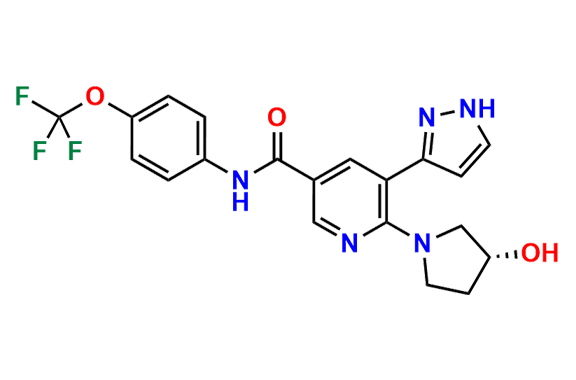
Asciminib
| CAT. No. | CP-A213000 |
|---|---|
| CAS. No. | 1492952-76-7 |
| Mol. F. | C20H18ClF2N5O3 |
| Mol. Wt. | 449.84 |
| Stock Status | Custom Synthesis |
| Rel. Cas No | 2119669-71-3 (HCl salt) ; 2679814-62-9 (HBr salt) |
- Synonyms: NA
- Chemical Name: (R)-N-(4-(Chlorodifluoromethoxy)phenyl)-6-(3-hydroxypyrrolidin-1-yl)-5-(1H-pyrazol-3-yl)nicotinamide