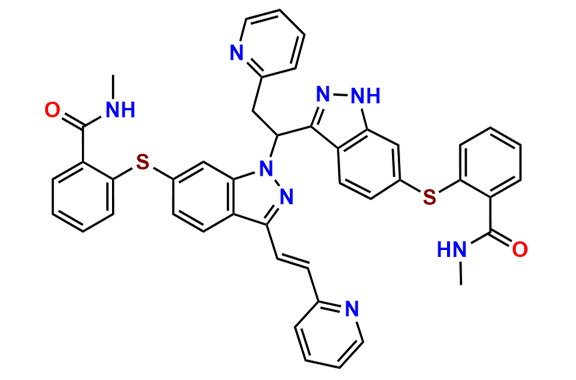
Axitinib Dimer
| CAT. No. | CP-A34001 |
|---|---|
| CAS. No. | 1428728-84-0 |
| Mol. F. | C44H36N8O2S2 |
| Mol. Wt. | 772.94 |
| Stock Status | Custom Synthesis |
- Category: Impurity Standards
- Synonyms: Asymmetric axitinib dimer impurity
- Chemical Name: N-Methyl-2-[[1-[1-[6-[[2-[(methylamino)carbonyl]phenyl]thio]-1H-indazol-3-yl]-2-(2-pyridinyl)ethyl]-3-[(1Z)-2-(2-pyridinyl)ethenyl]-1H-indazol-6-yl]thio]benzamide
FAQ
Axitinib Dimer is an impurity that may form during the synthesis, storage, or handling of Axitinib, a pharmaceutical compound used in cancer treatment.
Regulatory bodies like the FDA and EMA require monitoring of impurities like Axitinib Dimer to ensure the safety and efficacy of Axitinib formulations.
It forms due to interactions under conditions of heat, moisture, or acidity during the synthesis or storage of Axitinib.
By controlling storage conditions, managing pH levels, and routine testing, the formation of Axitinib Dimer can be minimized.
Techniques such as GC-MS, LC-MS, and HPLC-MS are commonly used for the detection and analysis of Axitinib Dimer.
Chemicea provides COA, H-NMR, MASS, HPLC, and TGA reports as standard, with additional reports like CNMR, IR, UV, DEPT, Water content, and CHNS available upon request.
Yes, Chemicea’s documentation meets the standards required by major regulatory agencies including USFDA, EMA, ANVISA, TGA, and PMDA.
Axitinib Dimer is stable for shipping at room temperature. Specific storage conditions are provided in the Certificate of Analysis (COA).
Yes, Chemicea offers both standard and customized pack sizes to meet specific client requirements.
Chemicea supplies Axitinib Dimer as a reference standard, supporting regulatory compliance, method validation, and impurity analysis in Axitinib formulations.