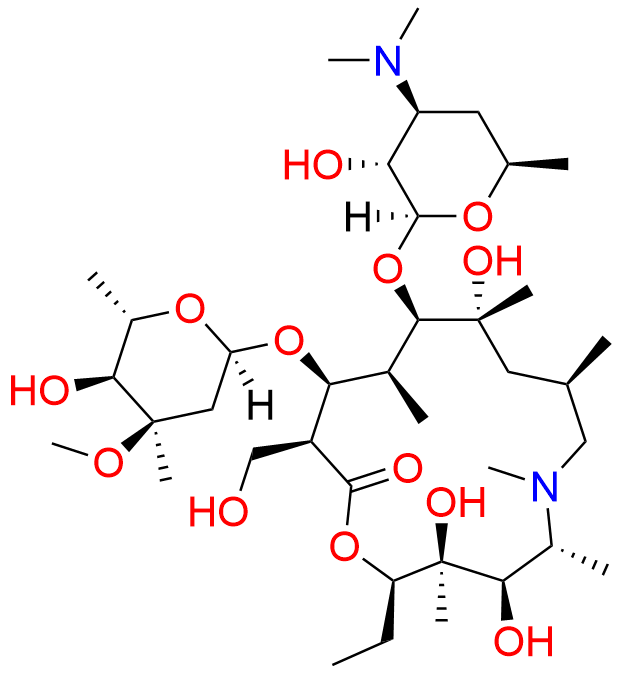
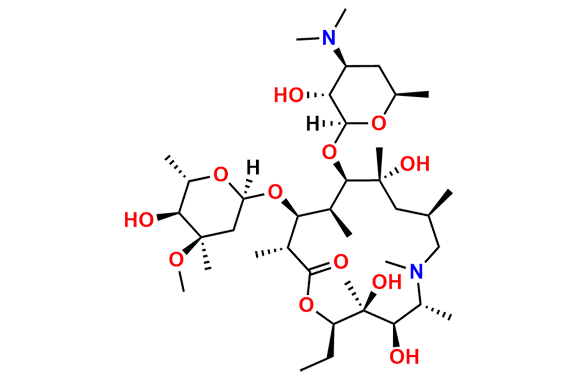
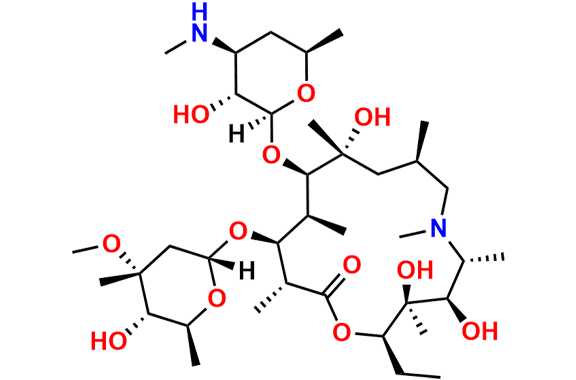
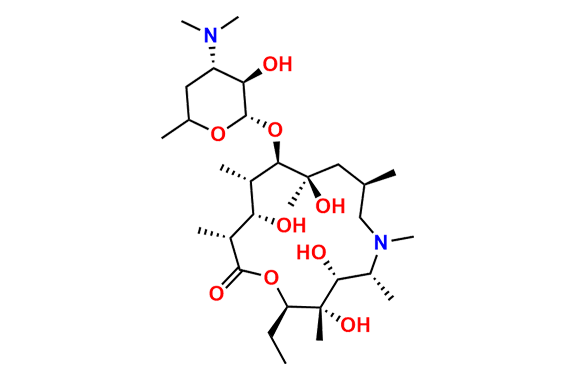
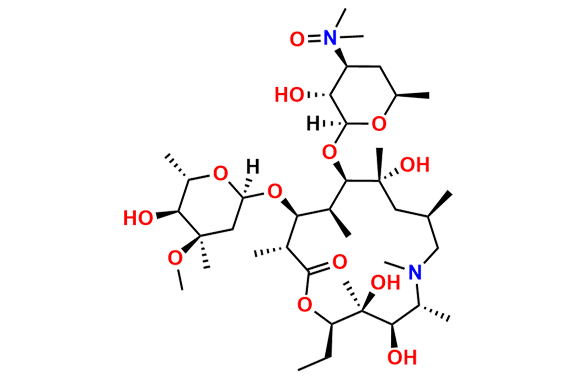
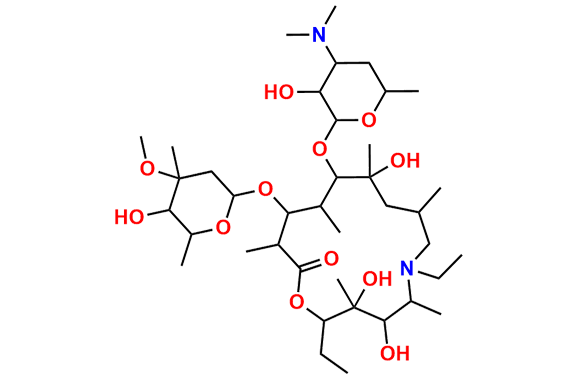
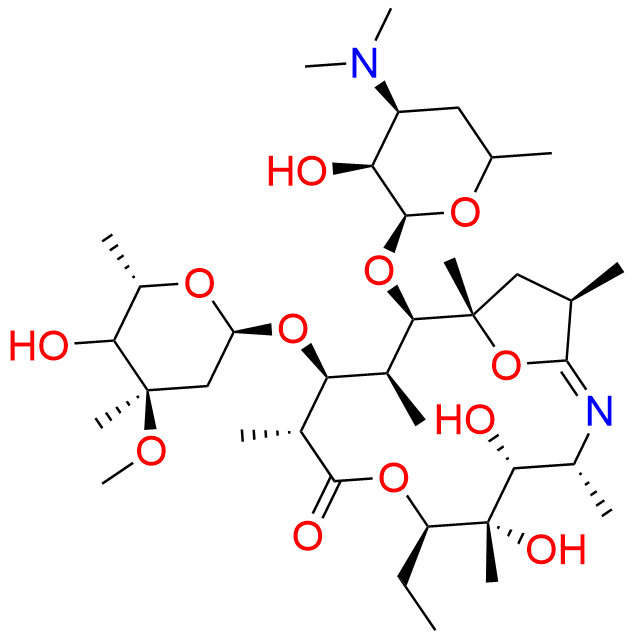
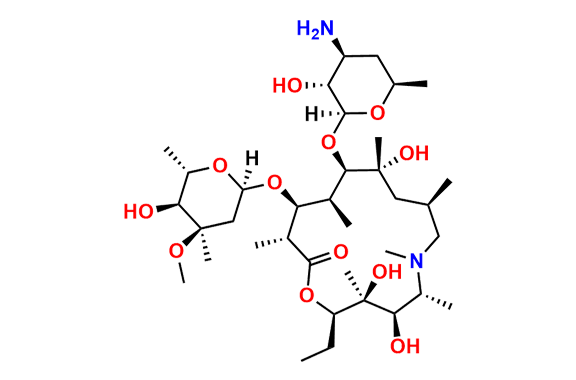
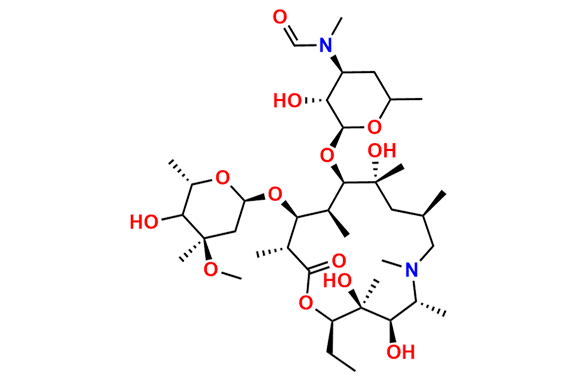
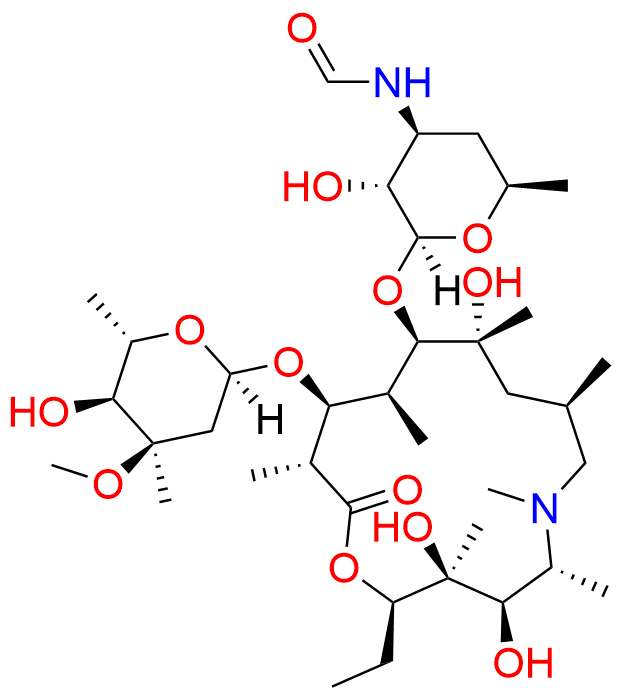
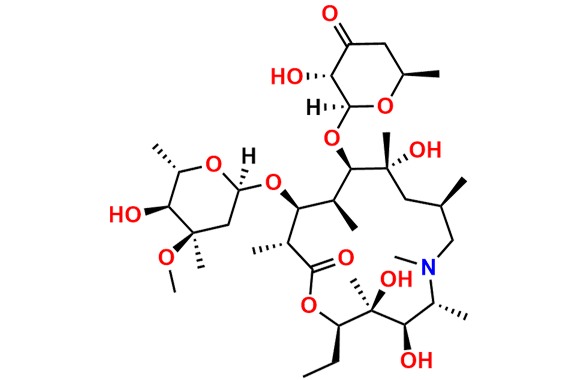
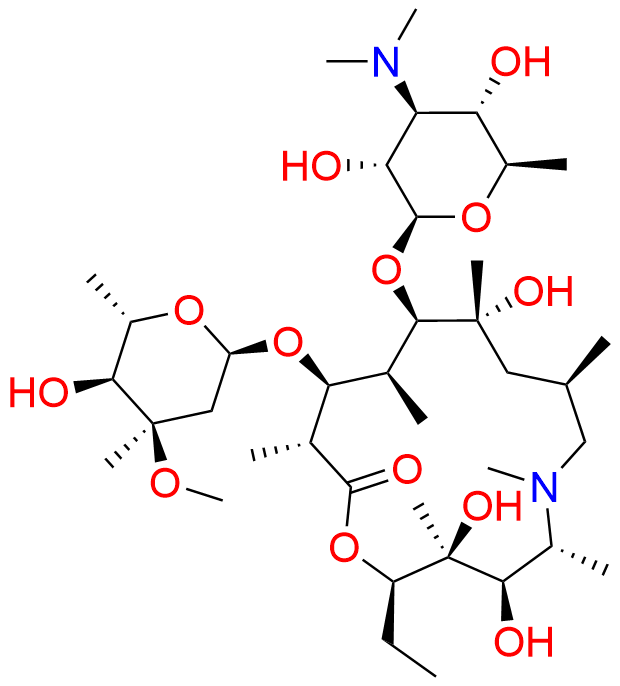
Azithromycin EP Impurity D
| CAT. No. | CP-A37004 |
|---|---|
| CAS. No. | 612069-26-8 |
| Mol. F. | C38H72N2O13 |
| Mol. Wt. | 765 |
| Stock Status | Custom Synthesis |
- Category: Impurity Standards
- Synonyms: Azithromycin F (EP)
- Chemical Name: 14-Demethyl-14-(hydroxymethyl)azithromycin (as per EP)