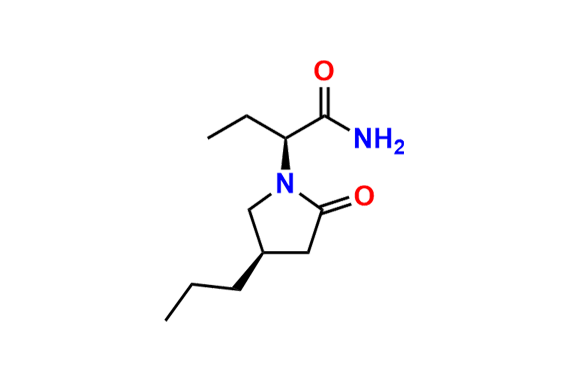
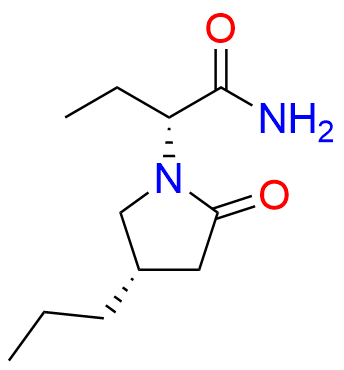
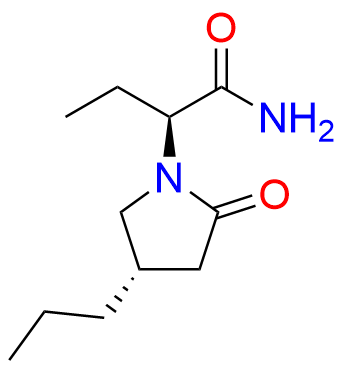
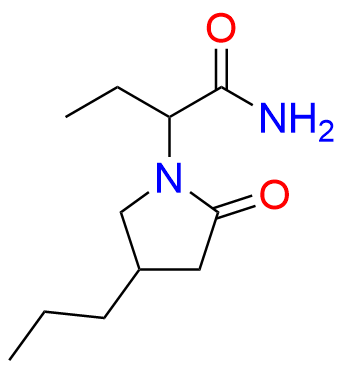
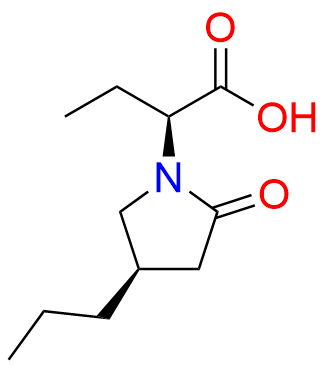
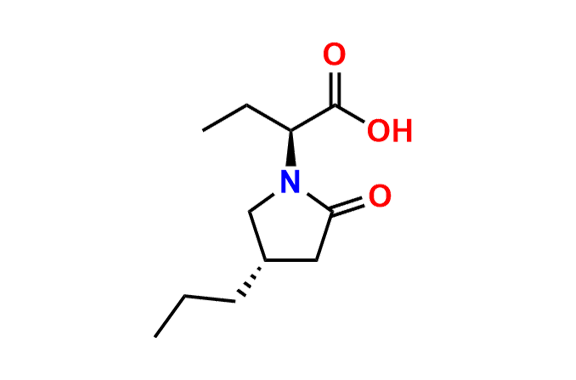
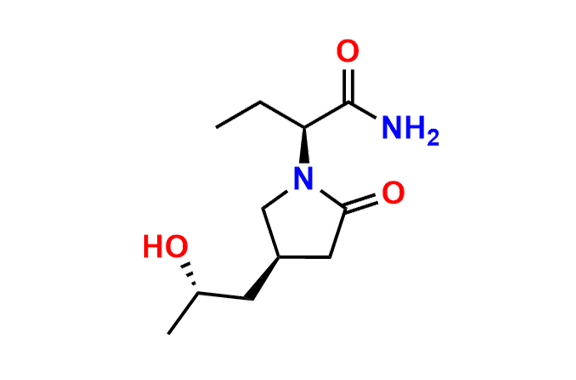
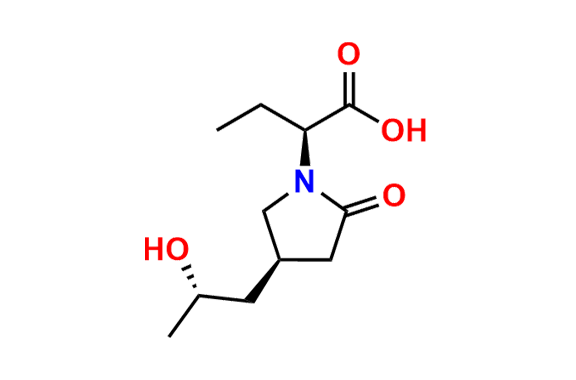
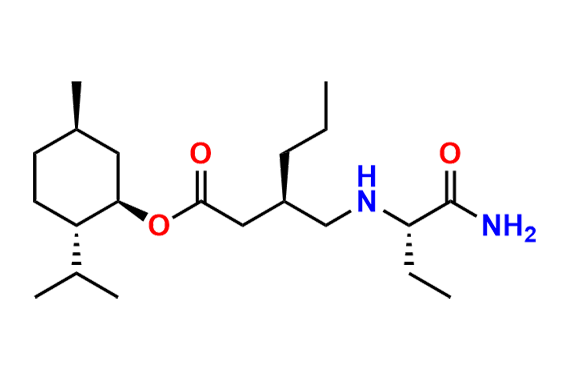
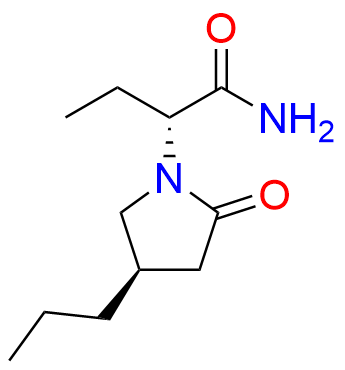
Brivaracetam (alfaR, 4R)-Isomer
| CAT. No. | CP-B35002 |
|---|---|
| CAS. No. | 357337-00-9 |
| Mol. F. | C11H20N2O2 |
| Mol. Wt. | 212.29 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: Brivaracetam EP Impurity C
- Chemical Name: (αR,4R)-α-Ethyl-2-oxo-4-propyl-1-pyrrolidineacetamide
FAQ
Brivaracetam Impurity B is an impurity that can occur in Brivaracetam formulations due to synthesis or storage conditions.
Regulatory bodies require monitoring impurities like Brivaracetam Impurity B to ensure the safety and efficacy of Brivaracetam formulations.
It forms as a by-product under conditions such as temperature and humidity variations during synthesis or storage.
By maintaining optimal storage conditions and conducting routine quality checks, the formation of this impurity can be managed.
Detection methods include GC-MS, LC-MS, and HPLC-MS for precise impurity profiling and analysis.
Chemicea provides COA, H-NMR, MASS, HPLC, and TGA reports, with additional reports like CNMR, IR, UV, DEPT, Water content, and CHNS available on request.
Yes, Chemicea’s documents meet the standards of major regulatory agencies such as the USFDA, EMA, ANVISA, TGA, and PMDA.
It is stable for shipping at room temperature. Specific storage conditions are outlined in the Certificate of Analysis (COA).
Yes, Chemicea offers both standard and customized pack sizes to meet client needs.
Chemicea supplies Brivaracetam Impurity B as a reference standard, supporting regulatory compliance, method validation, and impurity profiling.

 VIEW COA
VIEW COA