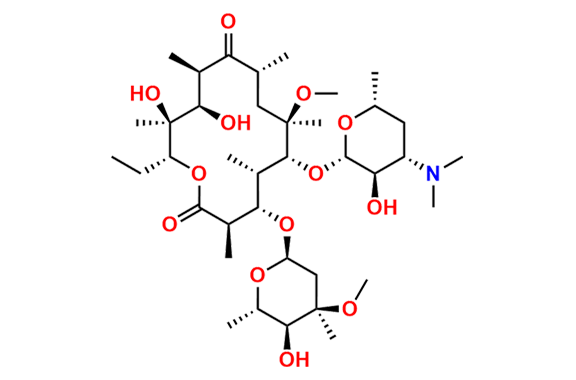
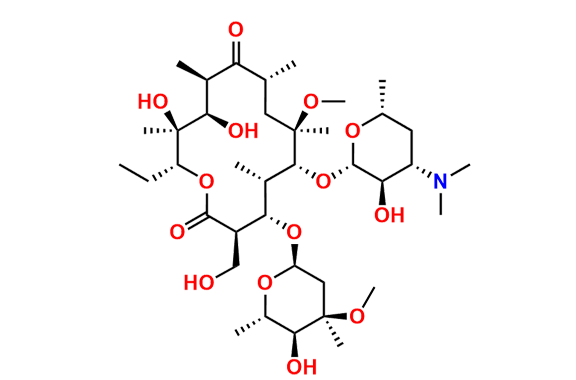
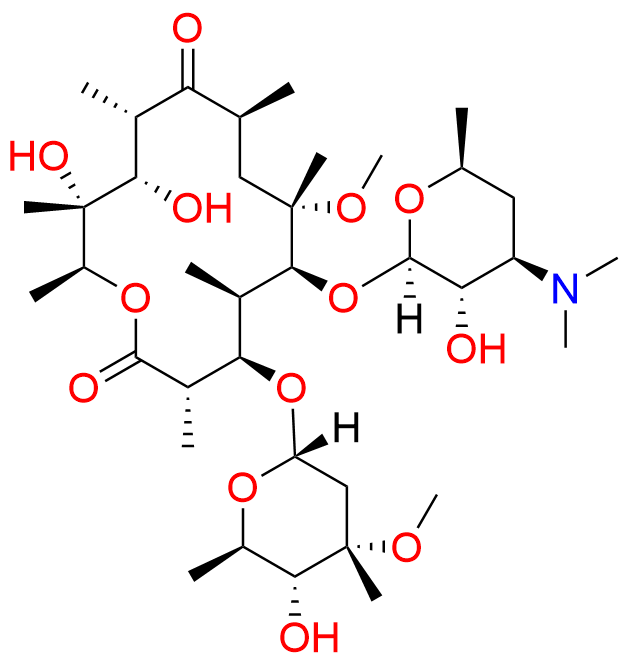
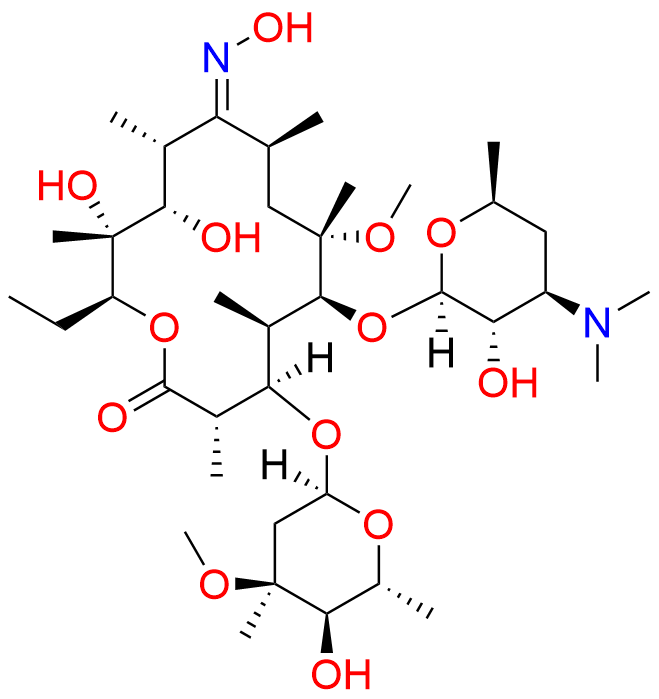
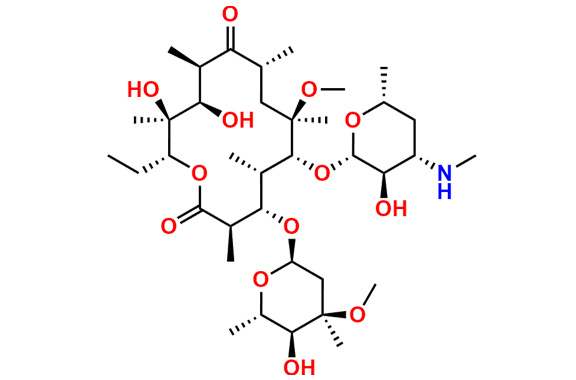
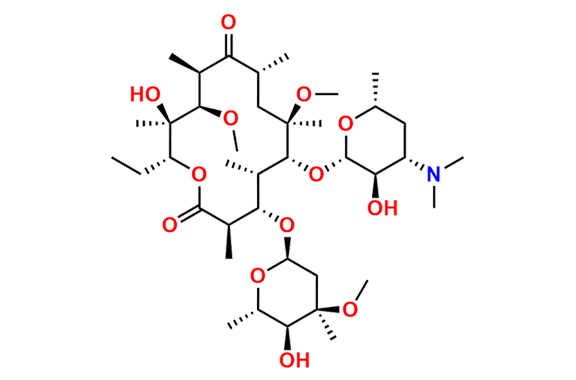
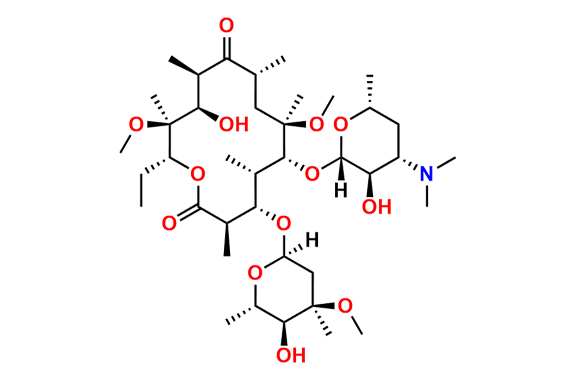
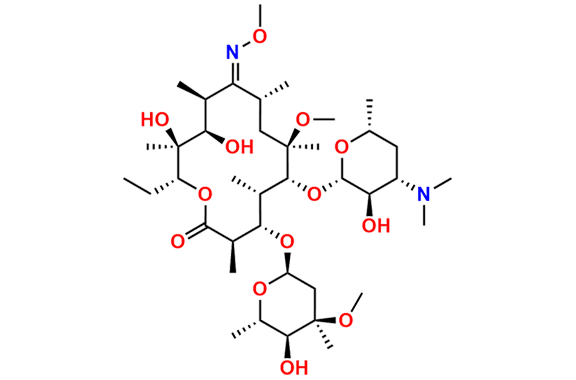
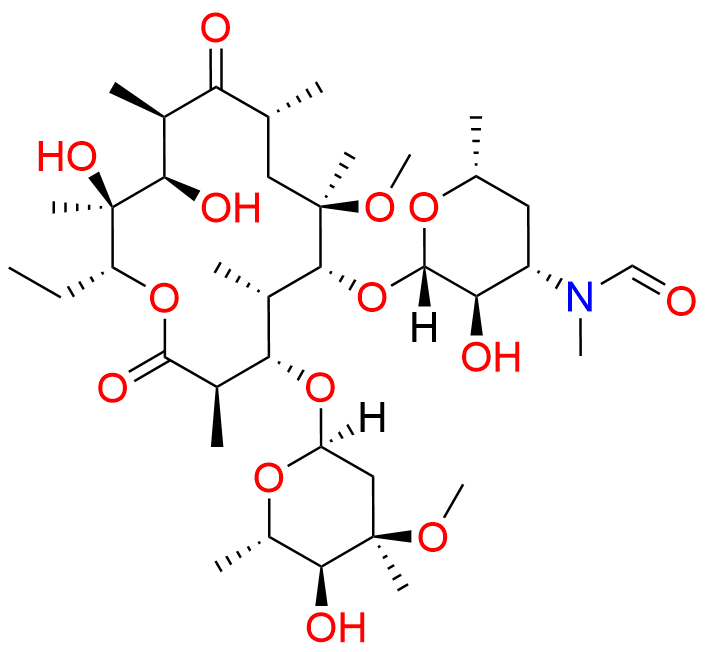
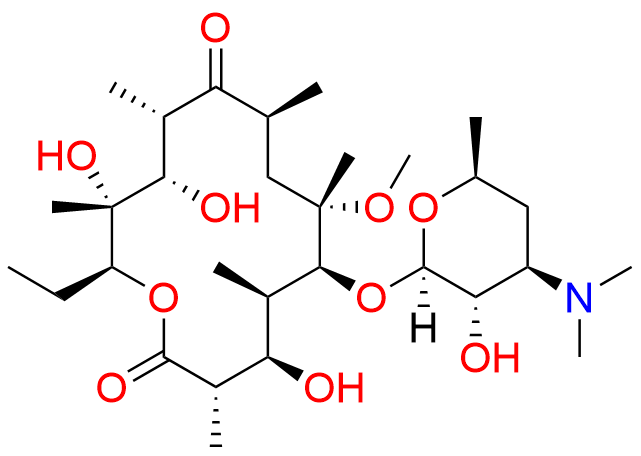
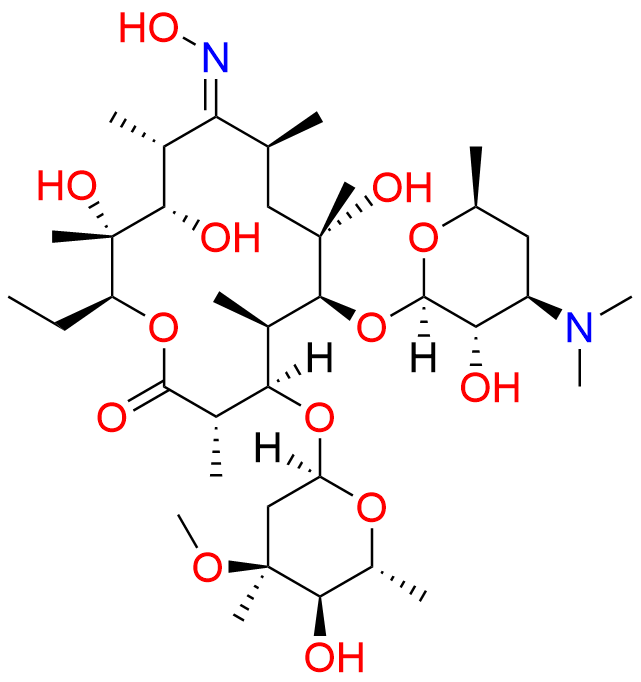
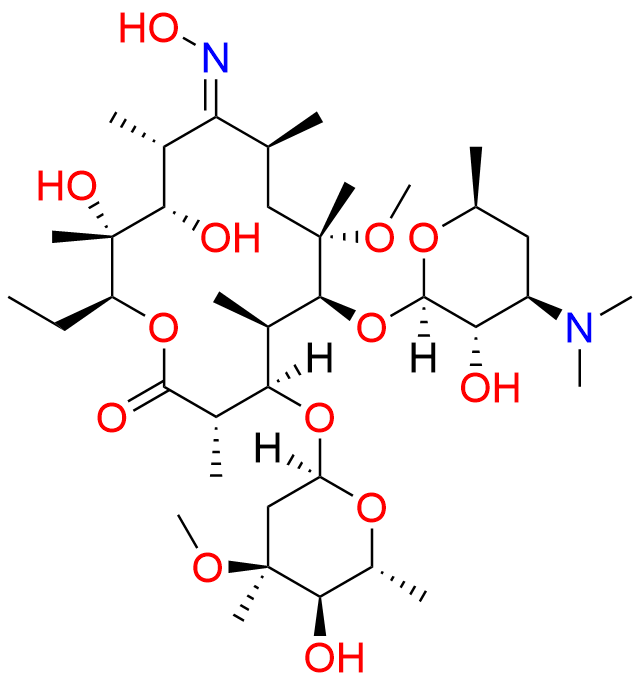
Clarithromycin EP Impurity L
| CAT. No. | CP-C1012 |
|---|---|
| CAS. No. | 127253-05-8 |
| Mol. F. | C38H70N2O13 |
| Mol. Wt. | 762.97 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: Clarithromycin(9Z)-Oxime
- Chemical Name: (3R,4S,5S,6R,7R,9R,11S,12R,13S,14R,Z)-6-(((2S,3R,4S,6R)-4-(Dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-14-ethyl-12,13-dihydroxy-4-(((2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-10-(hydroxyimino)-7-methoxy-3,5,7,9,11,13-hexamethyloxacyclotetradecan-2-one

 VIEW COA
VIEW COA