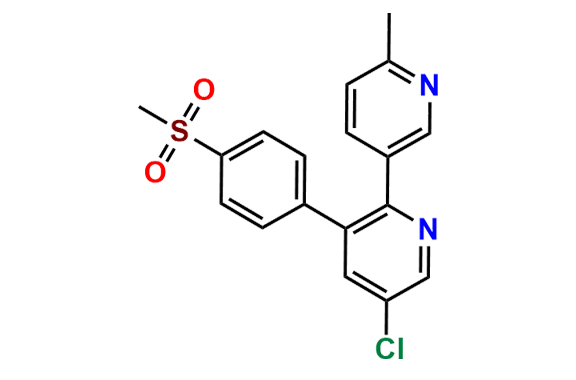
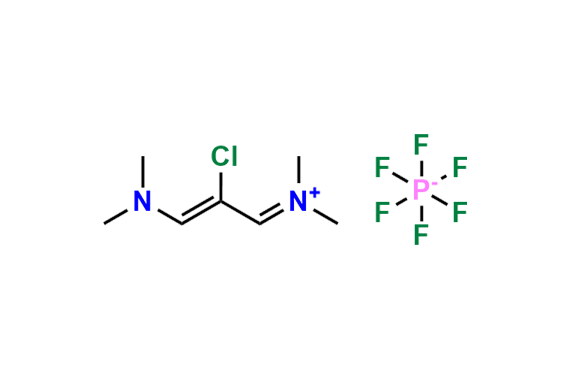
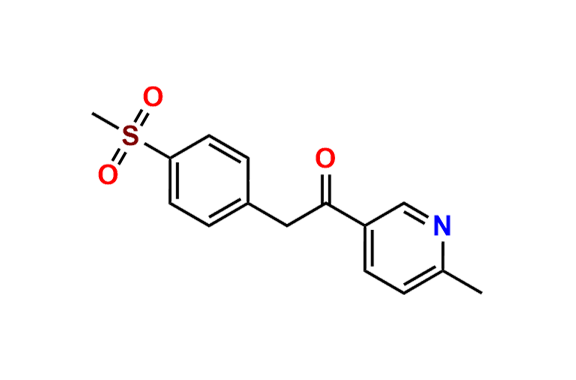
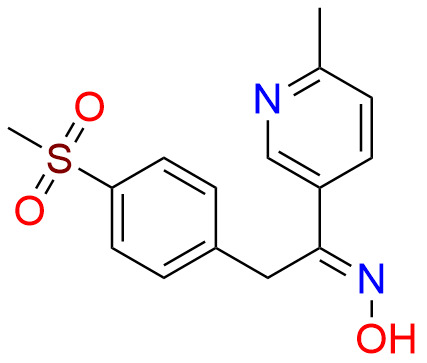
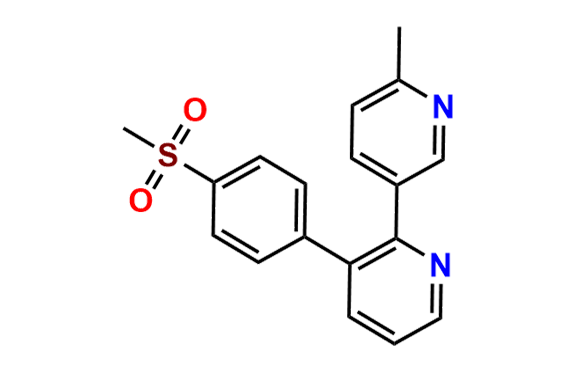
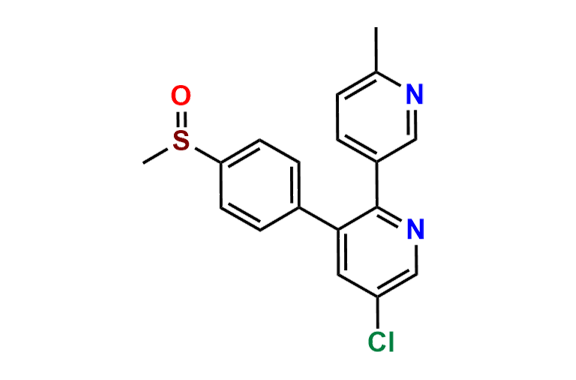
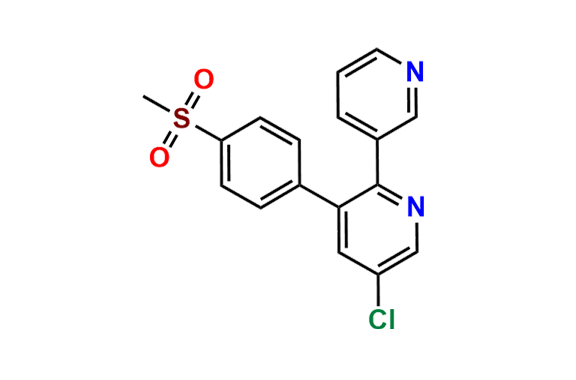
Etoricoxib Impurity 11
| CAT. No. | CP-E1001 |
|---|---|
| CAS. No. | 202409-31-2 |
| Mol. F. | C17H13ClN2O2S |
| Mol. Wt. | 344.82 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: Etoricoxib Desmethyl Impurity
- Chemical Name: 5-chloro-3-(4-(methylsulfonyl)phenyl)-2,3'-bipyridine

 VIEW COA
VIEW COA