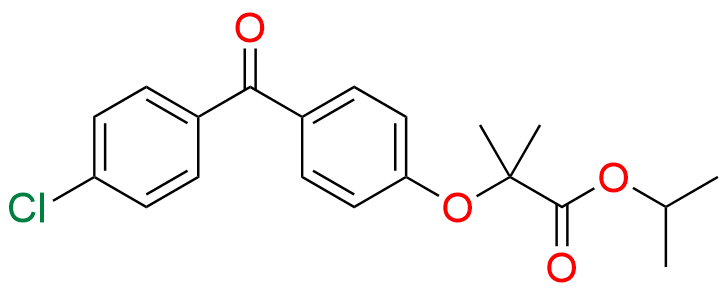
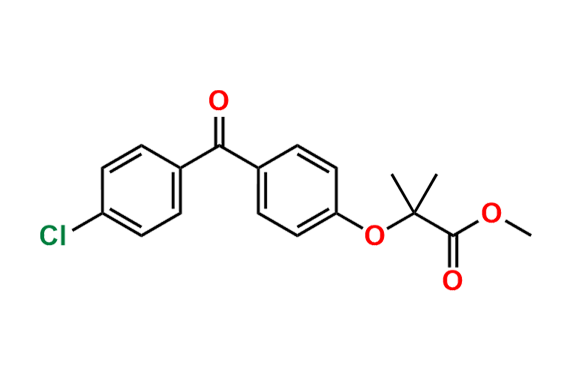
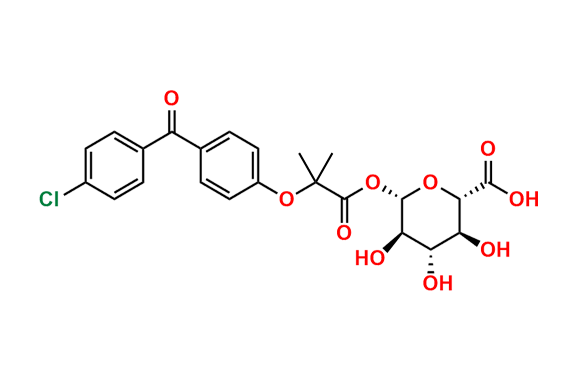
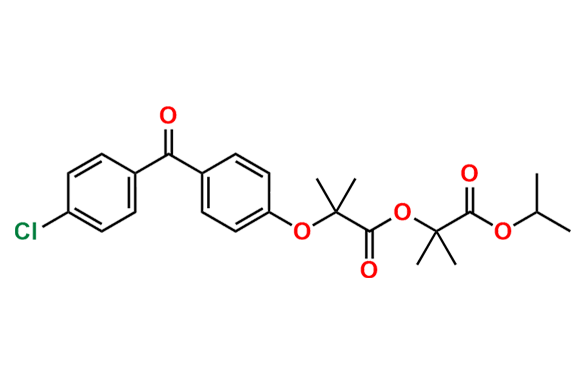
Fenofibrate EP Impurity G
| CAT. No. | CP-F12007 |
|---|---|
| CAS. No. | 217636-48-1 |
| Mol. F. | C24H27ClO6 |
| Mol. Wt. | 446.92 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: Fenofibrate USP Related Compound C
- Chemical Name: 1-Methylethyl 2-[[2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoyl]oxy]-2-methylpropanoate

 VIEW COA
VIEW COA