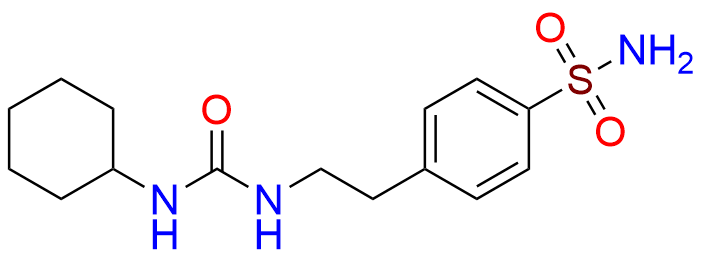
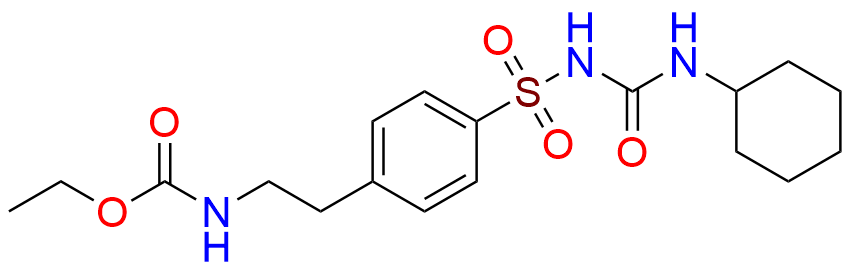
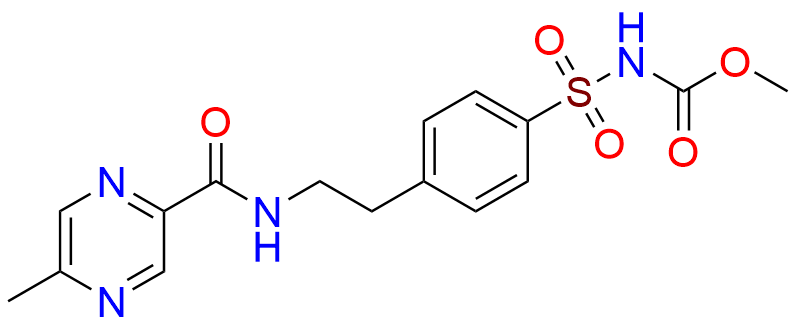
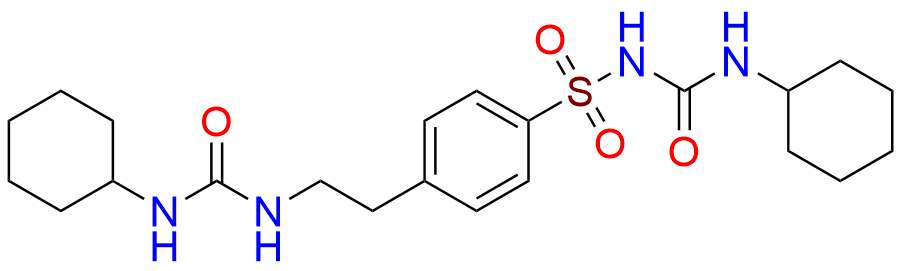
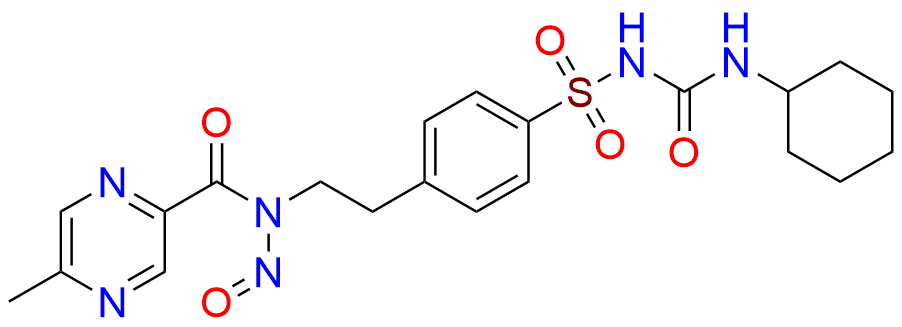
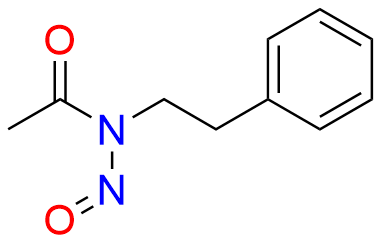
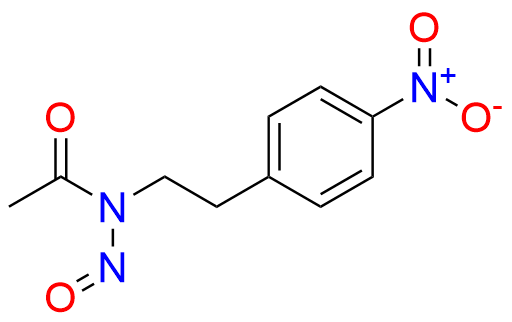
Glipizide EP Impurity H
| CAT. No. | CP-G11008 |
|---|---|
| CAS. No. | 10080-05-4 |
| Mol. F. | C15H23N3O3S |
| Mol. Wt. | 325.43 |
| Stock Status | Custom Synthesis |
- Category: Impurity Standards
- Synonyms: Glipizide BP Impurity H
- Chemical Name: 4-[2-[(Cyclohexylcarbamoyl)amino]ethyl]benzene-1-sulfonamide (as per EP)