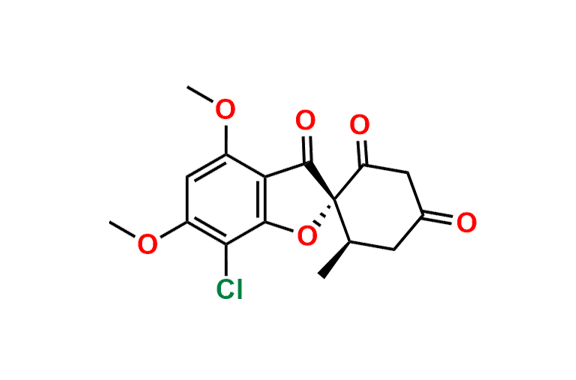
Griseofulvin EP Impurity A
| CAT. No. | CP-G17001 |
|---|---|
| CAS. No. | 469-54-5 |
| Mol. F. | C16H15ClO6 |
| Mol. Wt. | 338.74 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: Griseofulvic acid
- Chemical Name: (1′S,6′R)-7-Chloro-4,6-dimethoxy-6′-methyl-3H-spiro[1-benzofuran-2,1′-cyclohexane]-2′,3,4′-trione, (as per EP)
Griseofulvin EP Impurity A serves as an essential R&D reference material in the pharmaceutical sector. It is commonly employed in quality assurance processes, stability testing, and to meet regulatory requirements in Griseofulvin-related drug formulations. This impurity is integral to developing analytical methods, conducting toxicological evaluations, and calibrating laboratory instruments for accurate measurements.
Note: This product is exclusively intended for research and development purposes and is not suitable for animal or human consumption.

 VIEW COA
VIEW COA


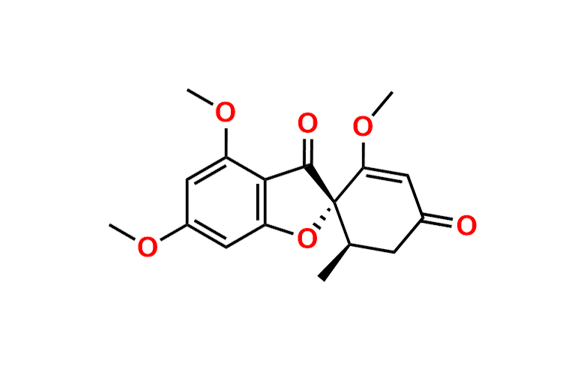
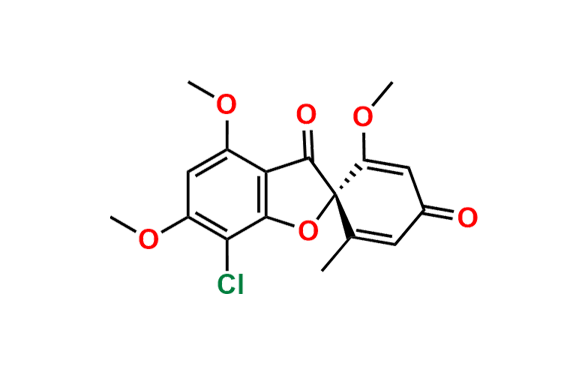
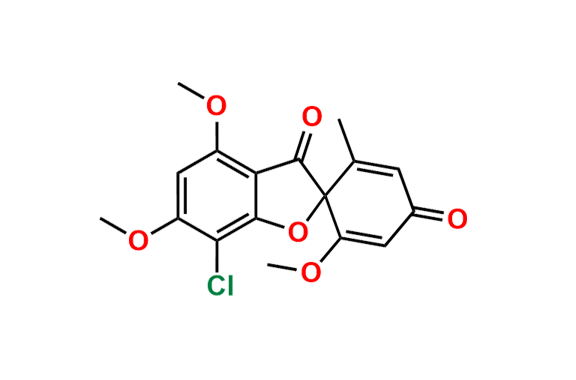
![(2S,2`R)-4,6-Dimethoxy-2`-methyl-3H-spiro[benzofuran-2,1`-cyclohexane]-3,4`,6`-trione](https://chemicea.com/admin/uploads/products/CP-G17005.png)