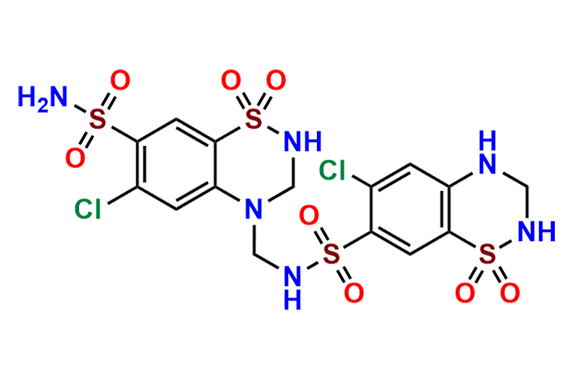
Hydrochlorothiazide EP impurity C
| CAT. No. | CP-H3003 |
|---|---|
| CAS. No. | 402824-96-8 |
| Mol. F. | C15H16Cl2N6O8S4 |
| Mol. Wt. | 607.47 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: Hydrochlorothiazide Dimer
- Chemical Name: 6-chloro-N-[(6-chloro-7-sulfamoyl-2,3-dihydro-4H-1,2,4-benzothiadiazin-4-yl 1,1-dioxide)methyl]-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide

 VIEW COA
VIEW COA