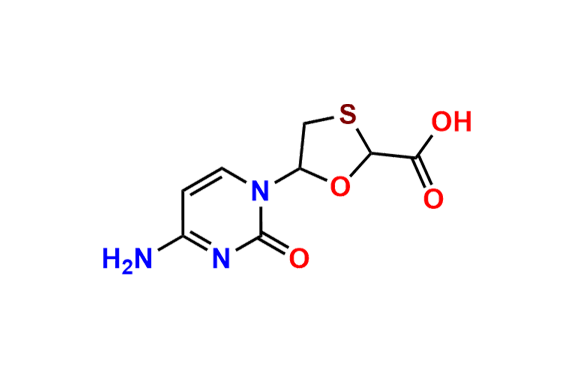
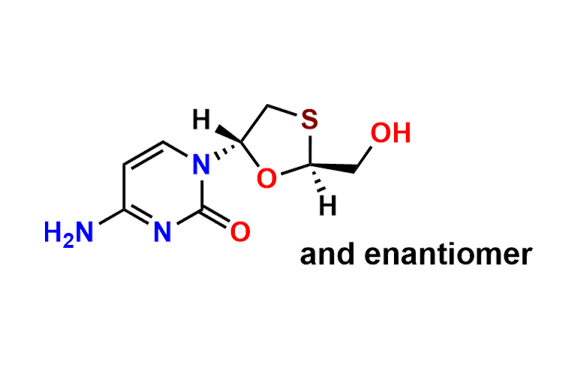
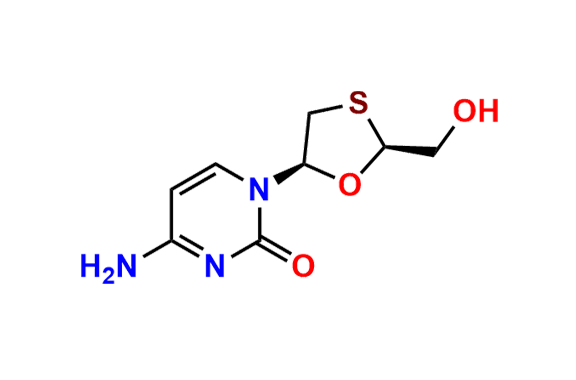
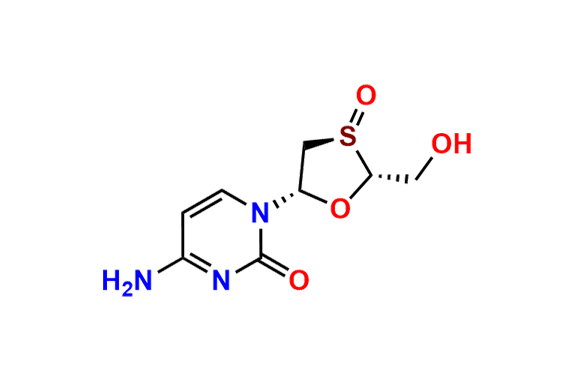
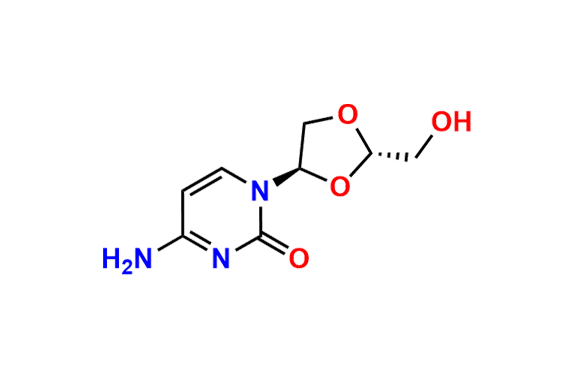
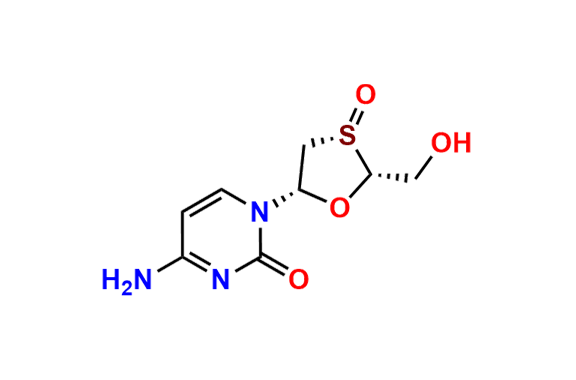
Lamivudine EP Impurity G
| CAT. No. | CP-L3007 |
|---|---|
| CAS. No. | 160552-55-6 |
| Mol. F. | C8H11N3O4S |
| Mol. Wt. | 245.25 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: Lamivudine S-sulfoxide
- Chemical Name: 4-Amino-1-((2R,3S,5S)-2-(hydroxymethyl)-3-oxido-1,3-oxathiolan-5-yl)pyrimidin-2(1H)-one
Lamivudine EP Impurity G is a vital reference standard used in the pharmaceutical industry for research and development (R&D). It is extensively employed in quality control procedures, stability testing, and to ensure compliance with regulatory standards in Lamivudine-related formulations. This impurity supports the development of analytical methods, toxicological assessments, and the calibration of laboratory equipment for precise and reliable measurements.
Note: This product is strictly intended for research and development purposes and is not suitable for animal or human use.

 VIEW COA
VIEW COA