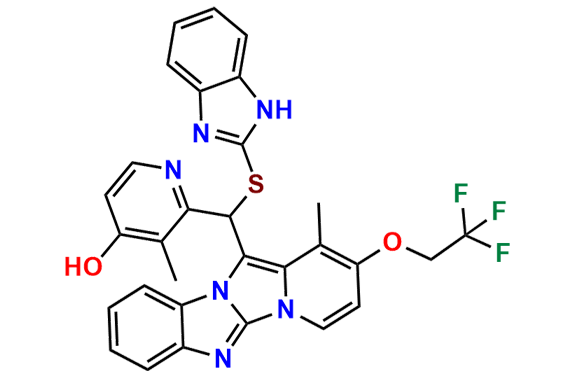
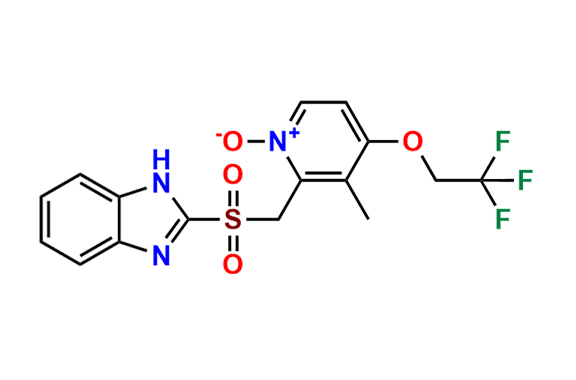
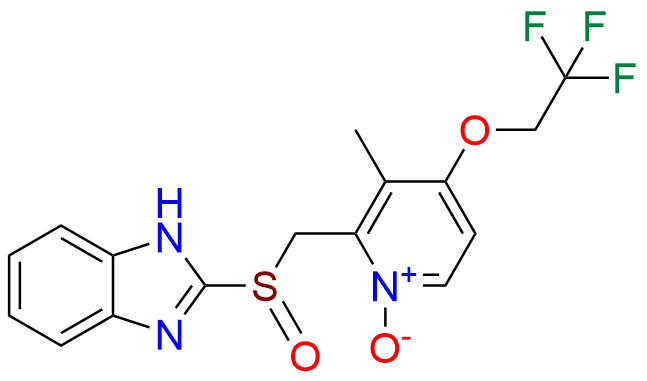
Lansoprazole Impurity 3
| CAT. No. | CP-L11044 |
|---|---|
| CAS. No. | NA |
| Mol. F. | C30H23F3N6O2S |
| Mol. Wt. | 588.6 |
| Stock Status | Custom Synthesis |
- Category: Impurity Standards
- Synonyms: NA
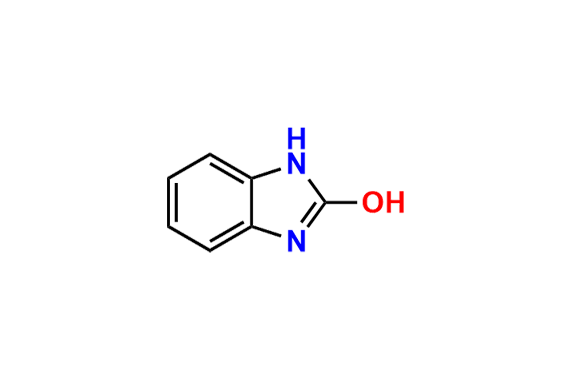
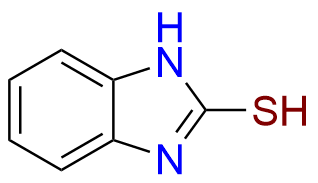
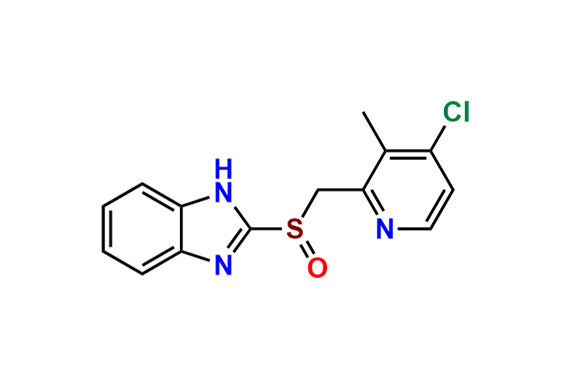
- Chemical Name: 2-(((1H-Benzo[d]imidazol-2-yl)thio)(1-methyl-2-(2,2,2-trifluoroethoxy)benzo[4',5']imidazo[2',1':2,3]imidazo[1,5-a]pyridin-12-yl)methyl)-3-methylpyridin-4-ol
Lansoprazole Impurity 3 plays a vital role in pharmaceutical research and development. It is widely used as a reference standard in quality control laboratories for monitoring impurity levels in active pharmaceutical ingredients (APIs) and drug formulations. This impurity supports stability studies by assessing API degradation under various conditions, ensuring product safety and efficacy. Its detailed impurity profiling is crucial for regulatory compliance in drug master files (DMFs) and Common Technical Documents (CTDs). Toxicological evaluations help establish safe impurity thresholds, while its detection enhances analytical methods like HPLC and LC-MS, driving innovation in impurity analysis and pharmaceutical quality assurance.
Note: Lansoprazole Impurity 3 is intended solely for research and development (R&D) purposes and is not suitable for human or animal consumption.