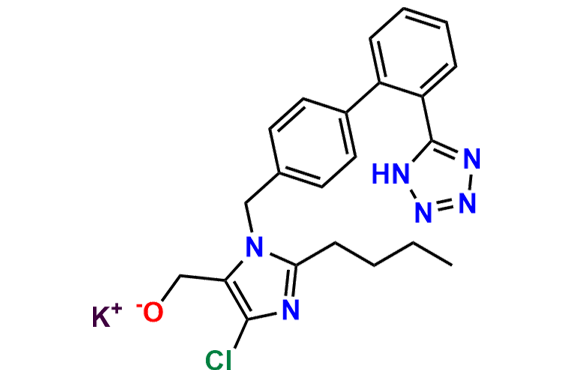
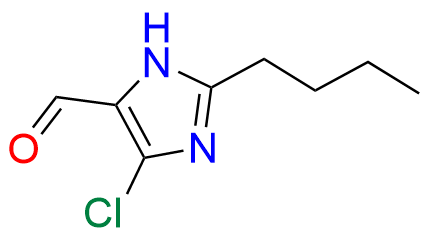
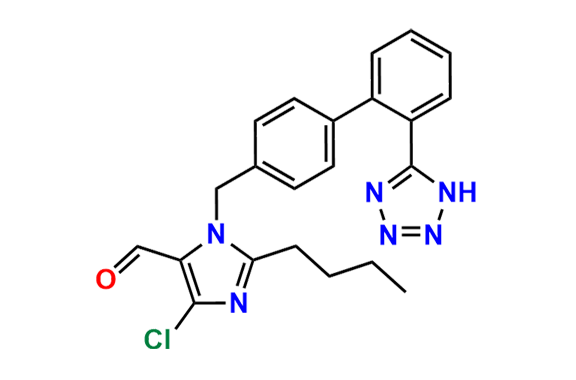
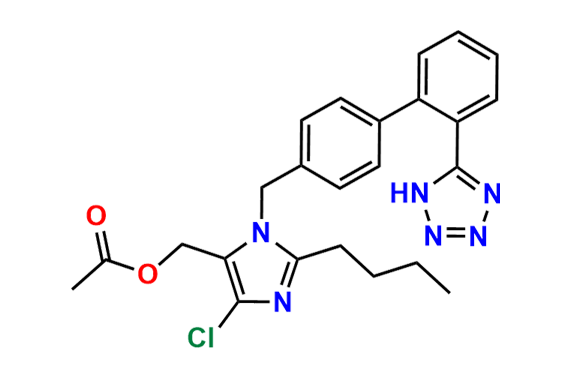
Losartan EP Impurity C
| CAT. No. | CP-L25003 |
|---|---|
| CAS. No. | 114799-13-2 |
| Mol. F. | C22H23ClN6O |
| Mol. Wt. | 422.92 |
| Stock Status | In Stock |
| Rel. Cas No | 860644-28-6 (K salt) |
- Category: Impurity Standards
- Synonyms: Isolosartan ; Losartan 5-Chloro Isomer
- Chemical Name: 2-Butyl-5-chloro-1-[[2′-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-1H-imidazol-4-yl]methanol (as per EP)

 VIEW COA
VIEW COA