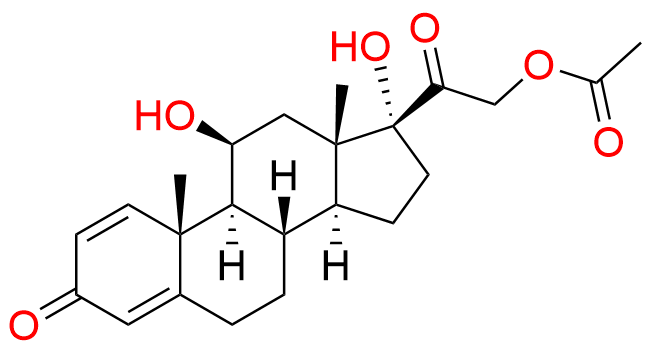
Methylprednisolone Acetate EP Impurity E
| CAT. No. | CP-M14025 |
|---|---|
| CAS. No. | 52-21-1 |
| Mol. F. | C23H30O6 |
| Mol. Wt. | 402.49 |
| Stock Status | Custom Synthesis |
- Category: Impurity Standards
- Synonyms: NA
- Chemical Name: 11b,17-dihydroxy-3,20-dioxopregna-1,4-dien-21-yl acetate