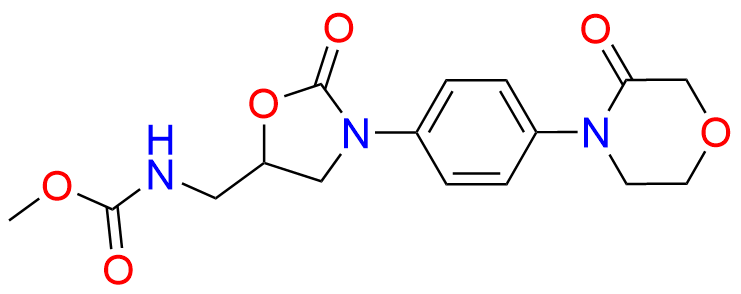
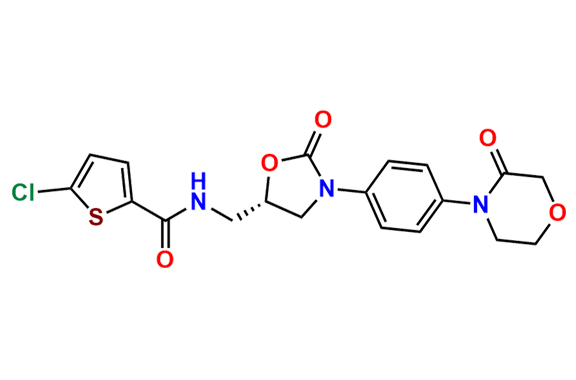
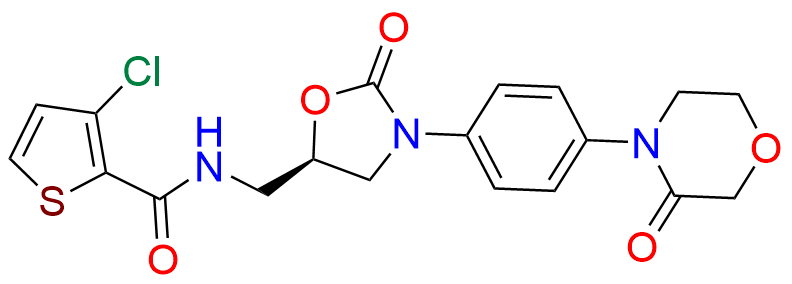
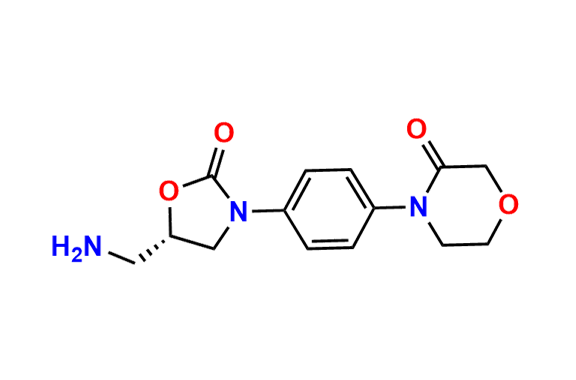
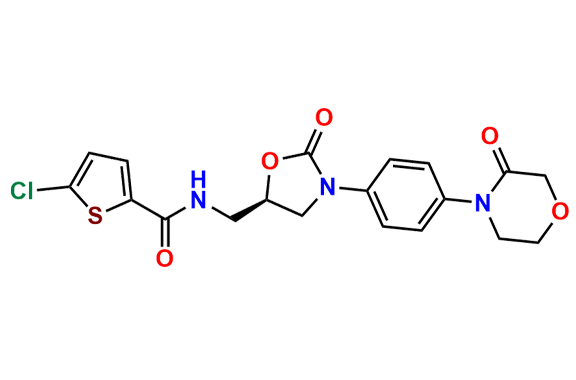
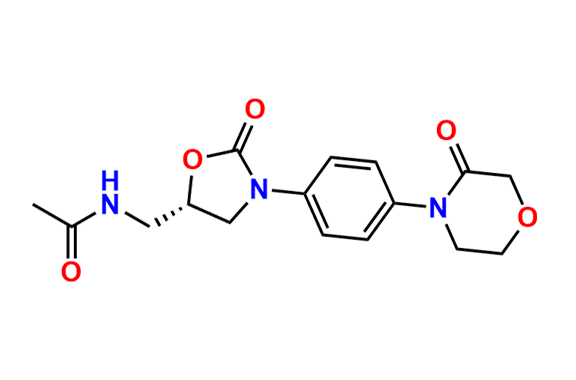
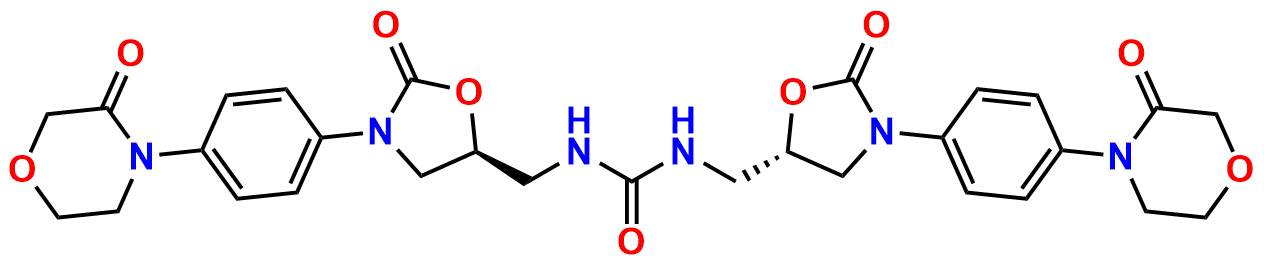
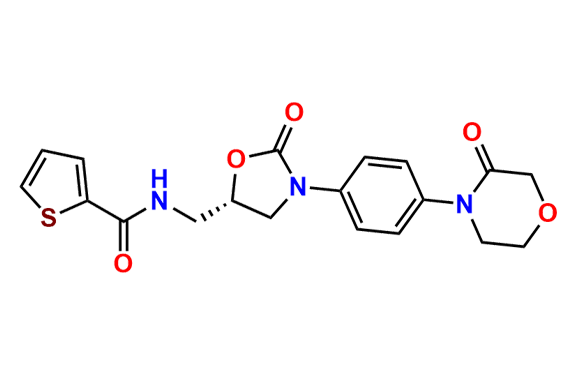
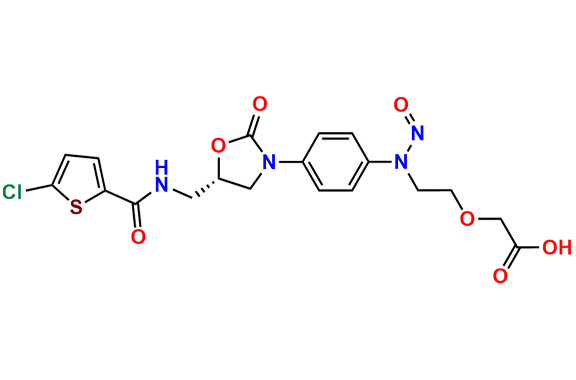
N-Nitroso Rivaroxaban Open-Ring Acid Impurity
| CAT. No. | CP-R17044 |
|---|---|
| CAS. No. | 3069765-41-6 |
| Mol. F. | C19H19ClN4O7S |
| Mol. Wt. | 482.89 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: NA
- Chemical Name: (S)-2-(2-((4-(5-((5-chlorothiophene-2-carboxamido)methyl)-2-oxooxazolidin-3-yl)phenyl)(nitroso)amino)ethoxy)acetic acid
FAQ
It is a nitrosamine impurity that can form during the synthesis, storage, or handling of Rivaroxaban, a medication used as an anticoagulant.
Nitrosamines, including N-Nitroso Rivaroxaban Open-Ring Acid Impurity, are regulated by agencies like the FDA and EMA due to potential health risks.
It forms when Rivaroxaban interacts with nitrosating agents, particularly under conditions involving heat, moisture, or acidity.
Formation can be managed by controlling storage conditions, managing pH levels, and conducting routine testing.
Advanced analytical methods such as GC-MS, LC-MS, and HPLC-MS are used for precise detection and quantification.
Chemicea supplies COA, H-NMR, MASS, HPLC, and TGA reports as standard, with additional reports like CNMR, IR, UV, DEPT, Water content, and CHNS available upon request.
Yes, they meet standards required by major agencies such as USFDA, EMA, ANVISA, TGA, PMDA, and others.
N-Nitroso Rivaroxaban Open-Ring Acid Impurity is stable for shipping at room temperature, with specific storage guidelines provided in the COA.
Yes, both standard and customized pack sizes are available to meet specific client needs.
Chemicea provides N-Nitroso Rivaroxaban Open-Ring Acid Impurity as a reference standard, supporting method validation, impurity analysis, and regulatory compliance.

 VIEW COA
VIEW COA