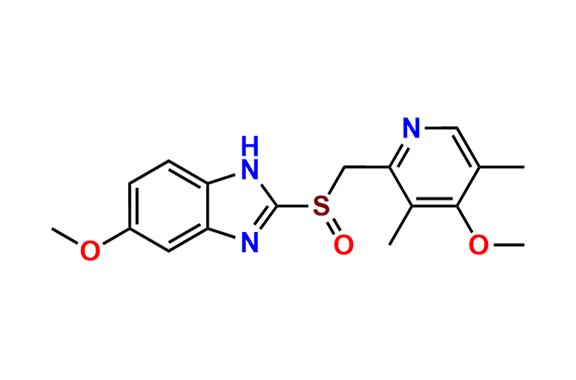
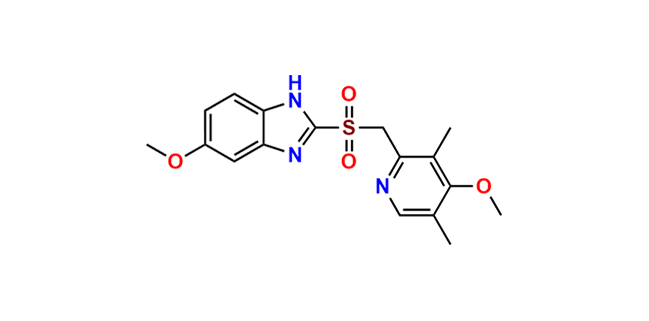
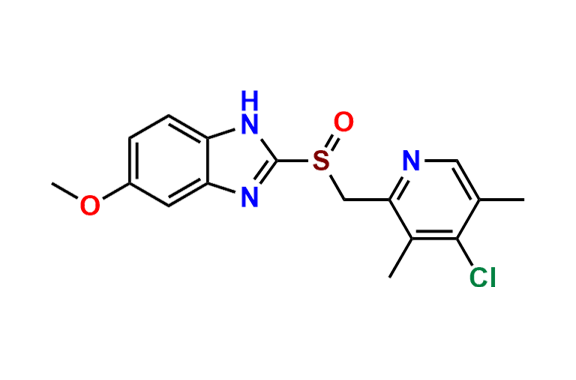
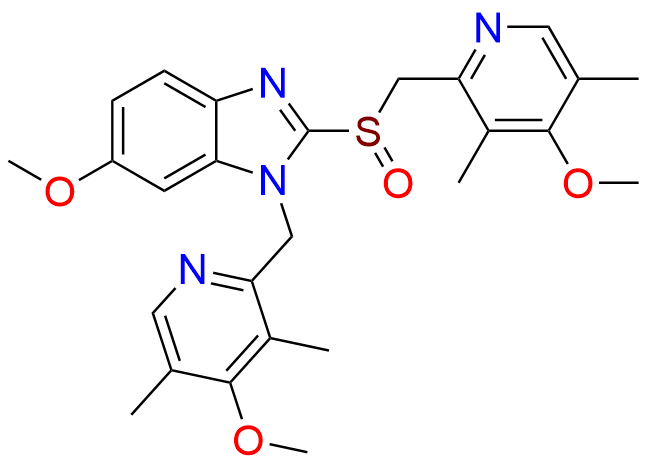
Omeprazole EP Impurity E
| CAT. No. | CP-O9005 |
|---|---|
| CAS. No. | 176219-04-8 |
| Mol. F. | C17H19N3O4S |
| Mol. Wt. | 361.42 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: Omeprazole BP Impurity E ; Omeprazole USP Related Compound E ; Esomeprazole EP Impurity E ; Omeprazole N-Oxide
- Chemical Name: 4-Methoxy-2-[[(RS)-(5-methoxy-1H-benzimidazol-2-yl)sulfinyl]methyl]-3,5-dimethylpyridine 1-Oxide

 VIEW COA
VIEW COA