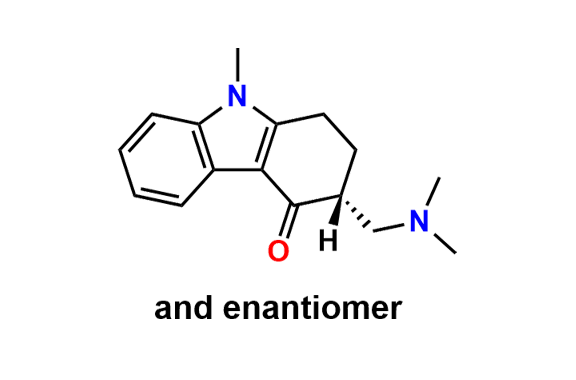
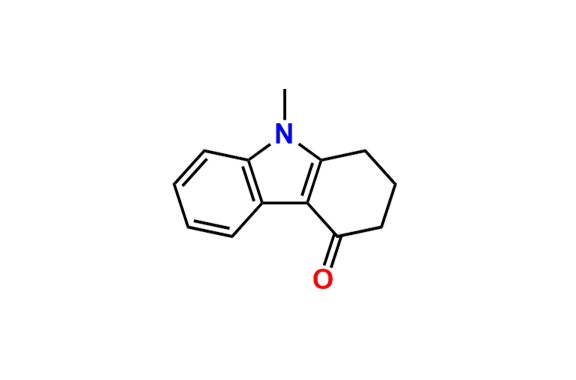
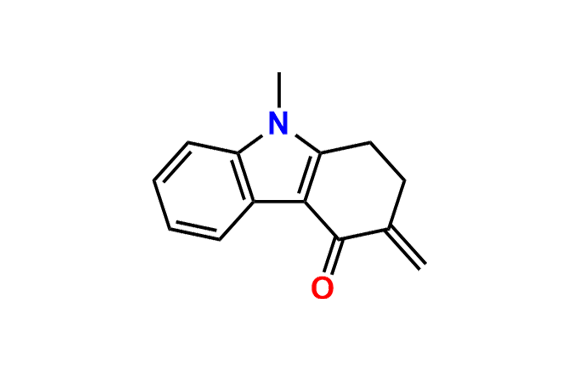
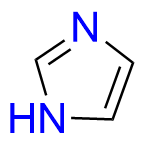
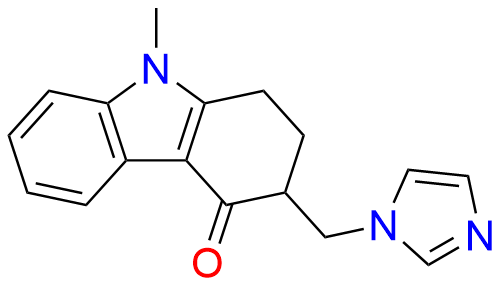
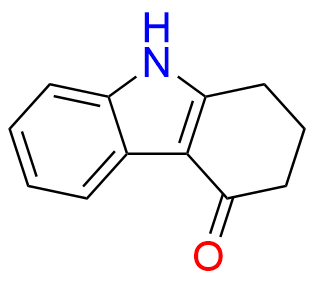
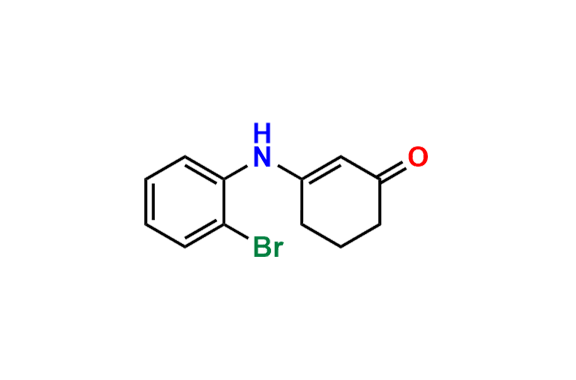
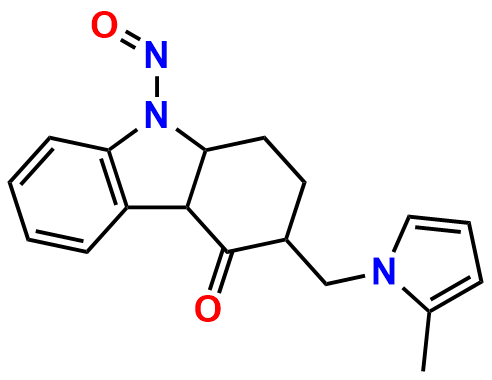
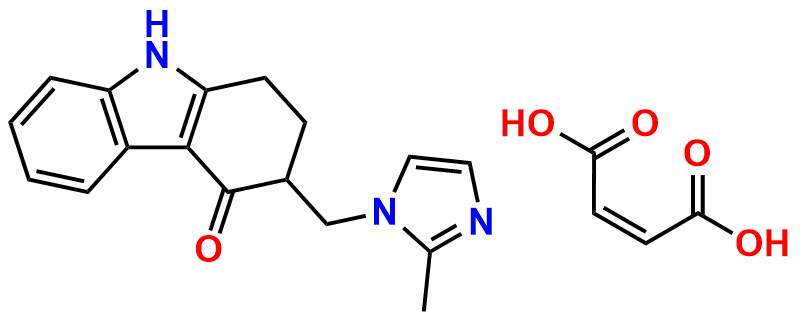
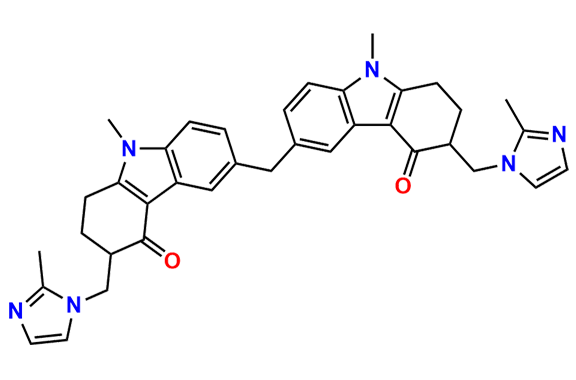
Ondansetron EP Impurity B
| CAT. No. | CP-O10002 |
|---|---|
| CAS. No. | 1076198-52-1 |
| Mol. F. | C37H38N6O2 |
| Mol. Wt. | 598.75 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: Ondansetron Dimer ; Methylene bisondansetron
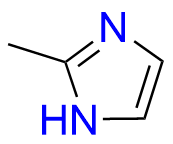
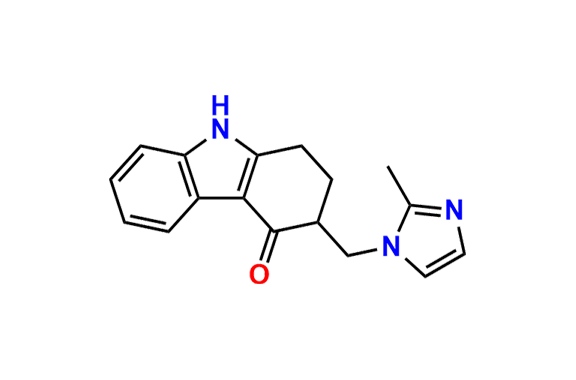
- Chemical Name: 6,6′-Methylenebis[(3Ξ)-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-1,2,3,9-tetrahydro-4H-carbazol-4-one] (as per EP)

 VIEW COA
VIEW COA