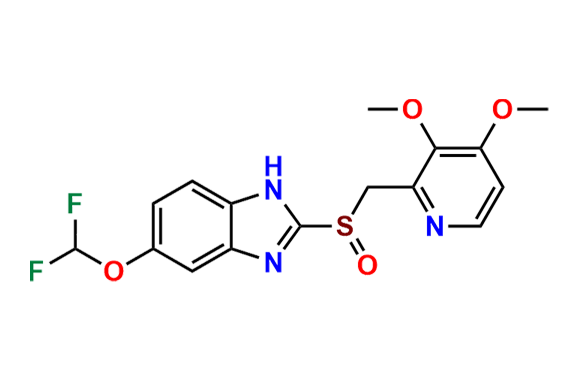
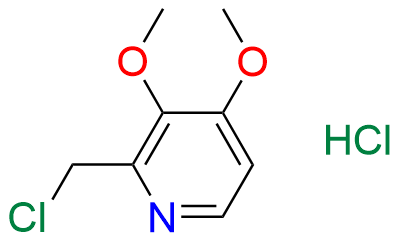
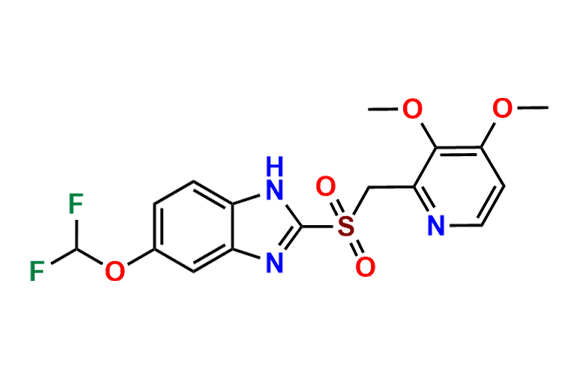
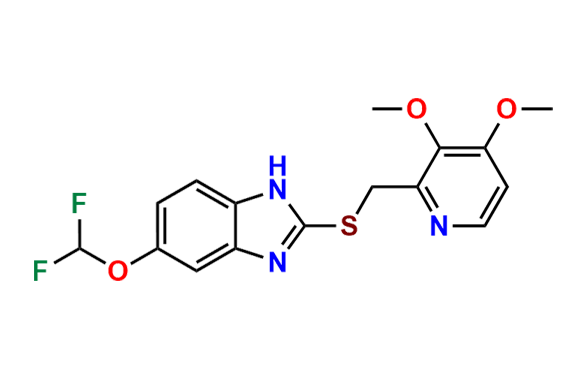
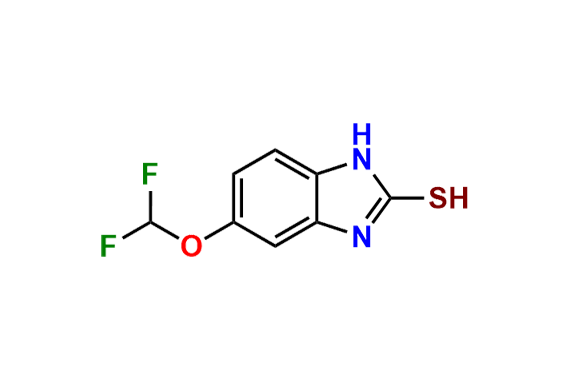
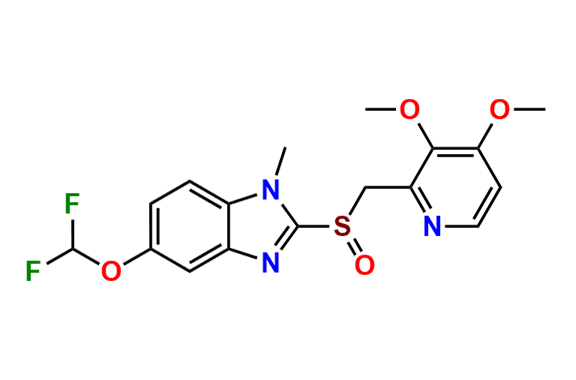
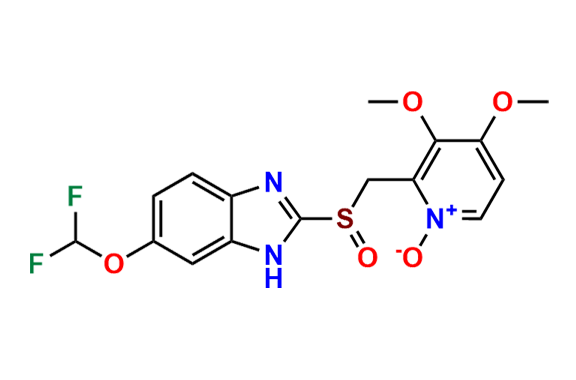
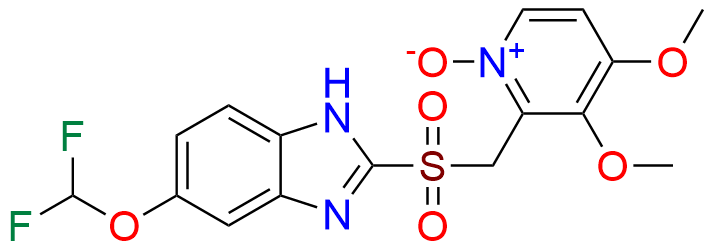
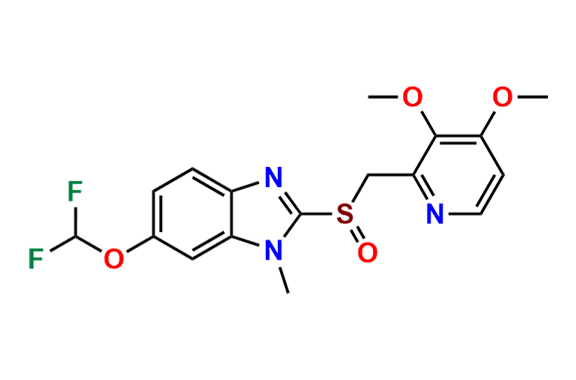
Pantoprazole EP Impurity D & F mixture
| CAT. No. | CP-P15010 |
|---|---|
| CAS. No. | NA |
| Mol. F. | C17H17F2N3O4S |
| Mol. Wt. | 397.40 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: Pantoprazole Related Compound D & F Mixture
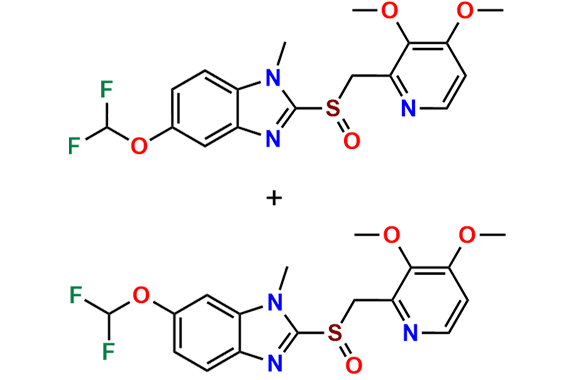
- Chemical Name: (A mixture consisting of 5-(difluoromethoxy)-2-[(RS)-[(3,4-dimethoxypyridin-2-yl)methyl]sulfinyl]-1-methyl-1Hbenzimidazole and 6-(difluoromethoxy)-2-[(RS)-[(3,4-dimethoxypyridin-2-yl)methyl]sulfinyl]-1-methyl-1Hbenzimidazole (as per USP)
FAQ
Pantoprazole EP Impurity D & F mixture is an impurity that may form in Pantoprazole formulations due to specific synthesis or storage conditions.
Monitoring this impurity is required by regulatory authorities to ensure the safety and efficacy of Pantoprazole formulations.
It forms under specific synthesis or storage conditions that can lead to impurity development in Pantoprazole.
Proper storage and careful control of synthesis conditions can help minimize its formation.
GC-MS, LC-MS, and HPLC-MS are commonly used to detect and quantify Pantoprazole EP Impurity D & F mixture accurately.
Chemicea provides COA, H-NMR, MASS, HPLC, and TGA reports as standard, with additional reports like CNMR, IR, UV, DEPT, Water content, and CHNS available upon request.
Yes, Chemicea’s documentation meets standards required by major regulatory bodies including USFDA, EMA, ANVISA, and PMDA.
It is stable for shipping at room temperature, with specific storage guidelines provided in the COA.
Yes, Chemicea offers both standard and customized pack sizes to meet specific client needs.
Chemicea supplies Pantoprazole EP Impurity D & F mixture as a reference standard, aiding regulatory compliance, method validation, and impurity profiling.

 VIEW COA
VIEW COA