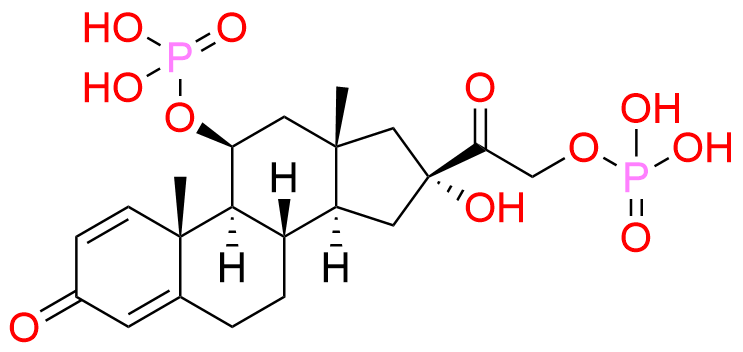
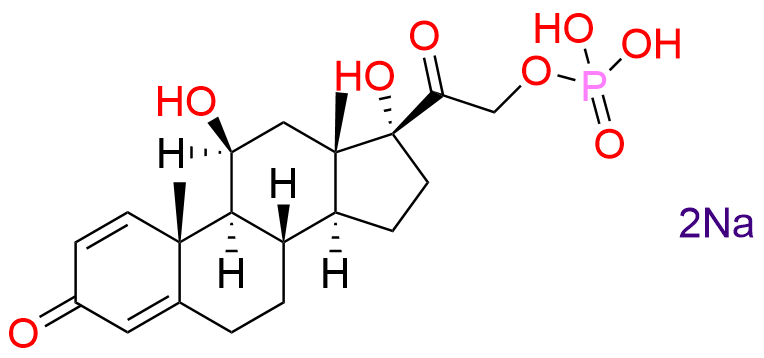
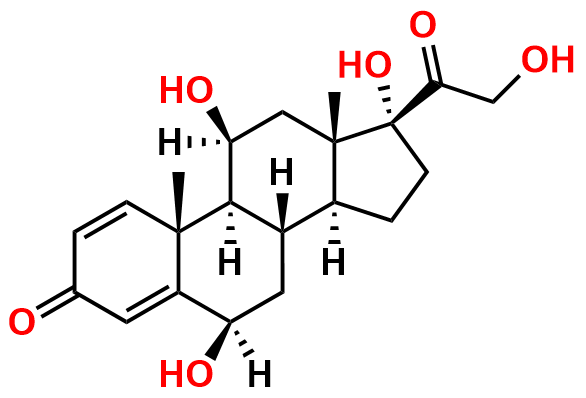
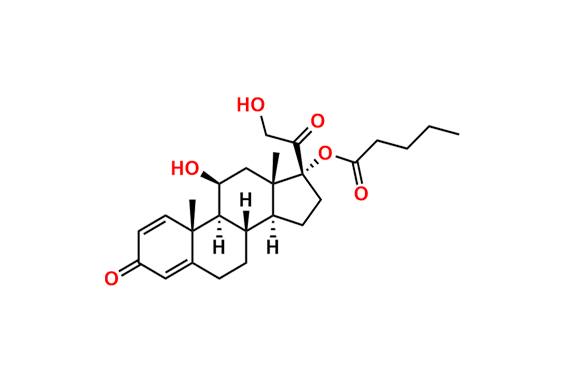
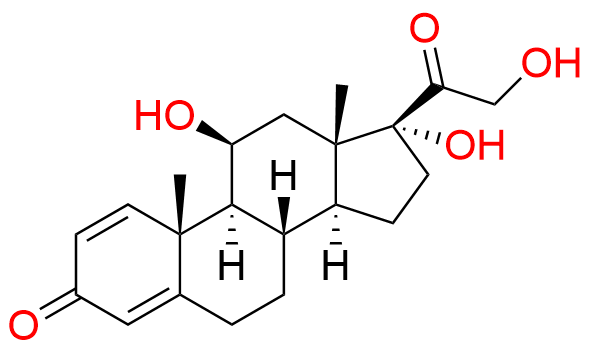
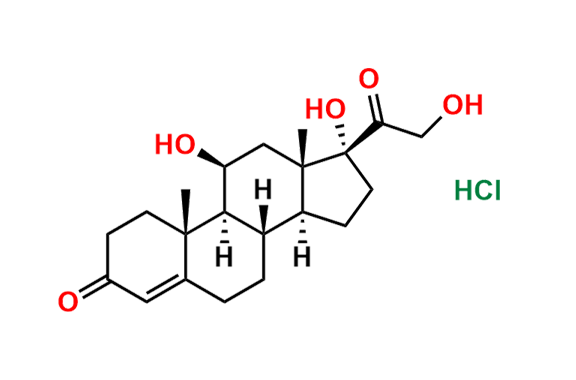
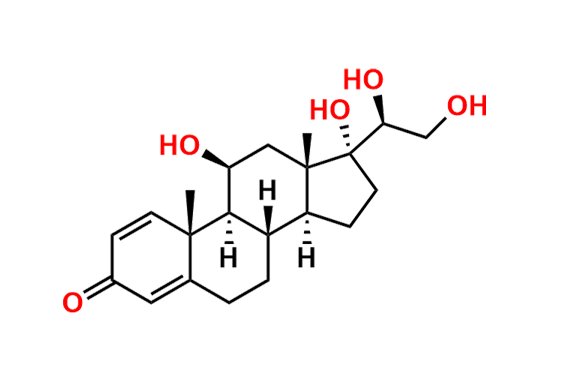
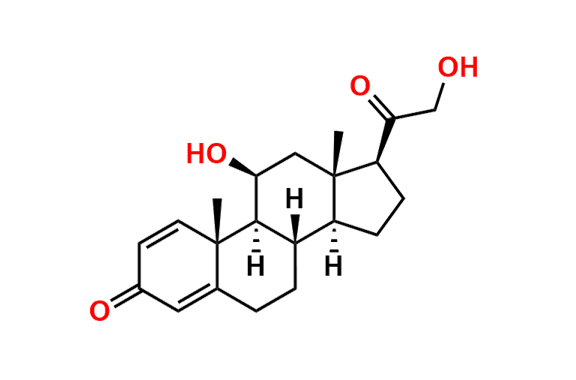
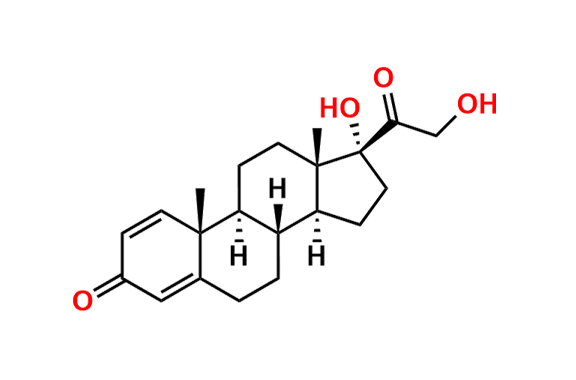
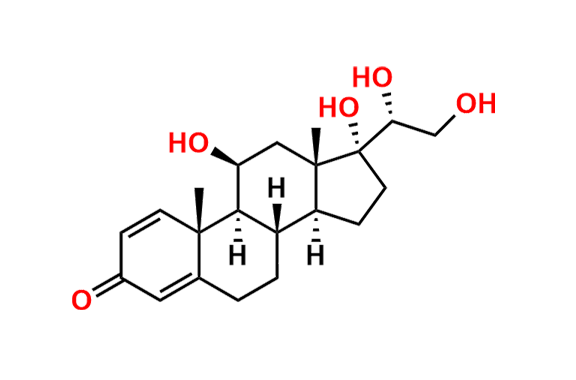
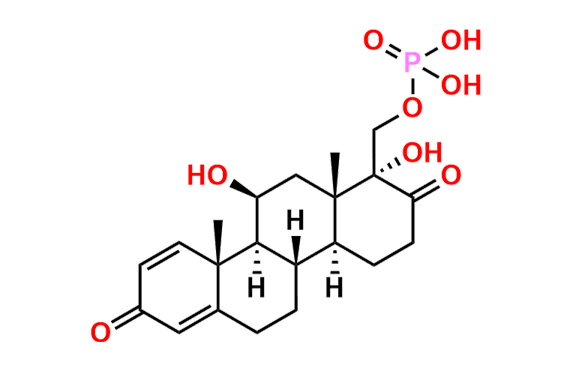
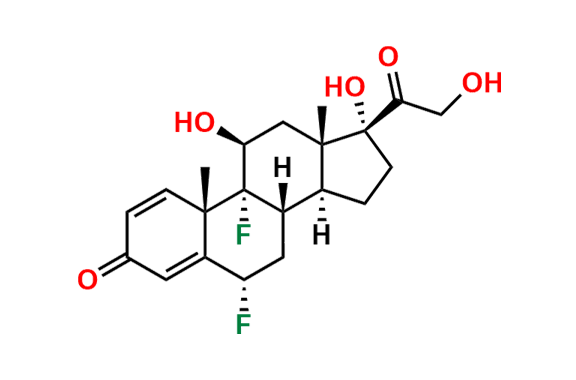
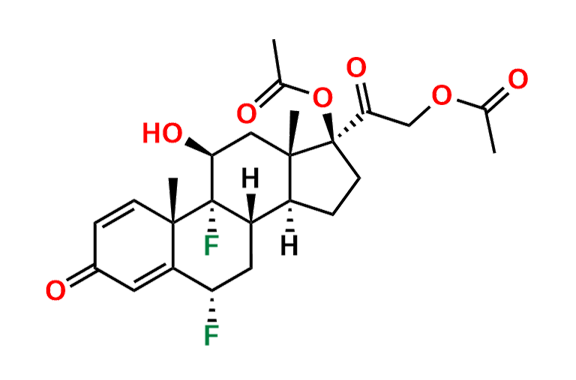
Prednisolone Sodium Phosphate USP Impurity E
| CAT. No. | CP-P74005 |
|---|---|
| CAS. No. | NA |
| Mol. F. | C21H30O11P2 |
| Mol. Wt. | 520.41 |
| Stock Status | Custom Synthesis |
- Category: Impurity Standards
- Synonyms: NA
- Chemical Name: 16β-Phosphoryloxyacetyl-11β,16α-dihydroxyandrosta-1,4-diene-3-one 11-phosphate (as per USP)