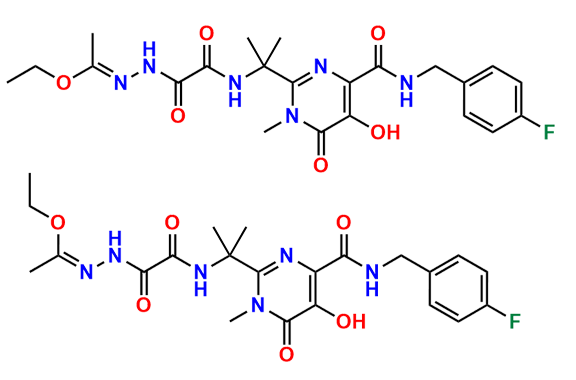
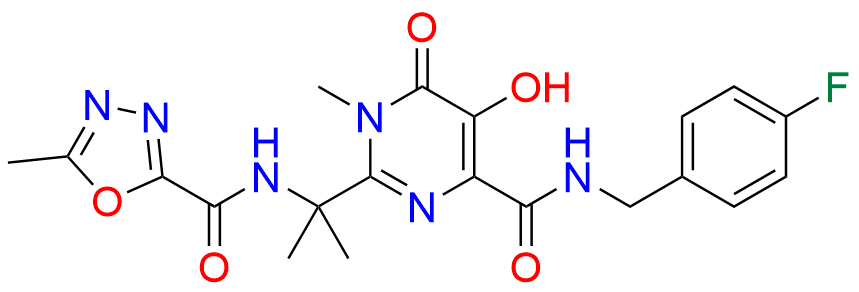
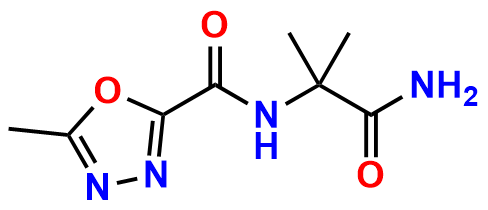
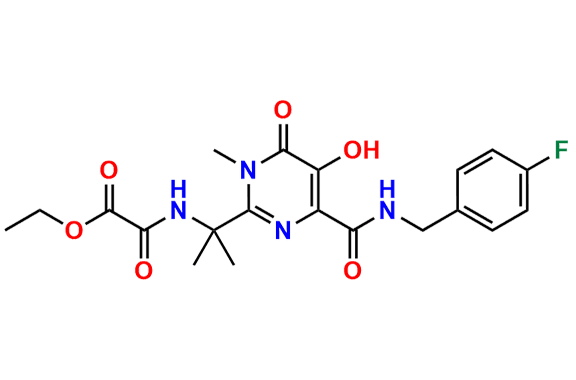
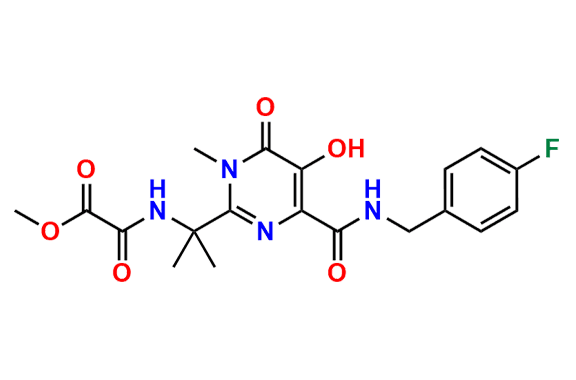
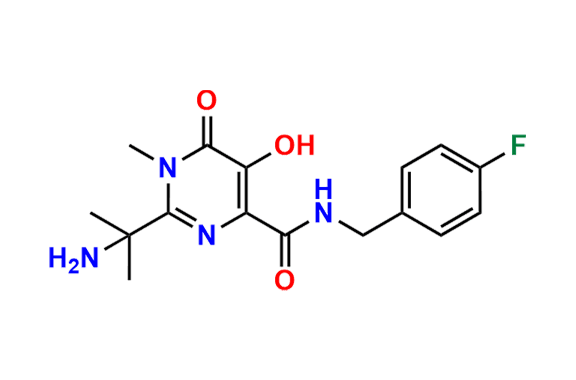
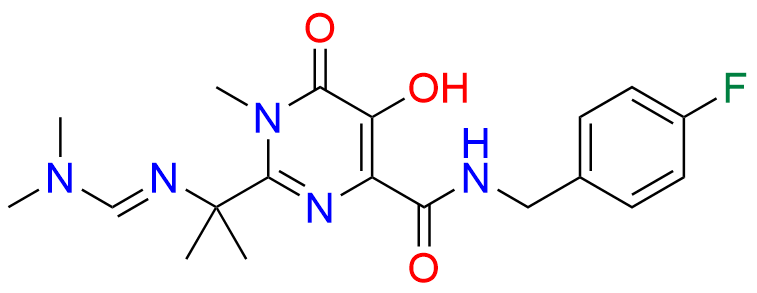
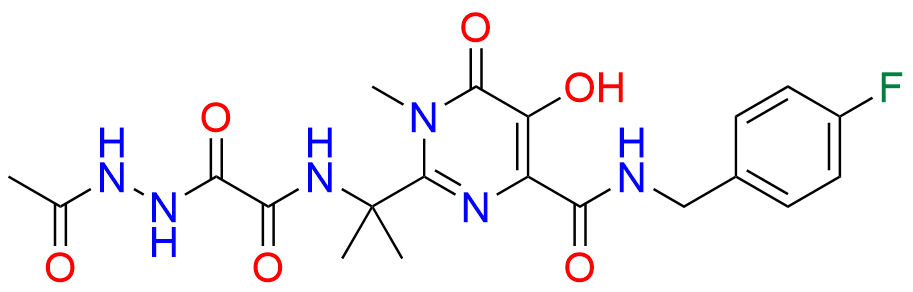
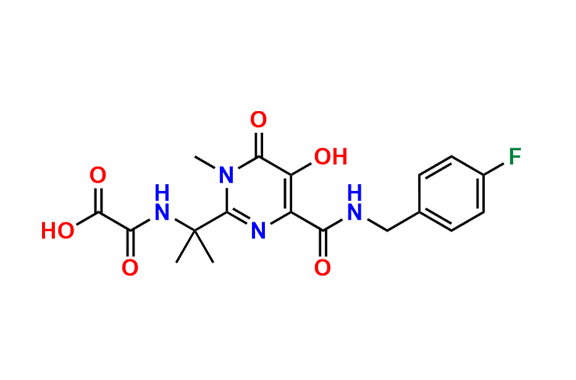
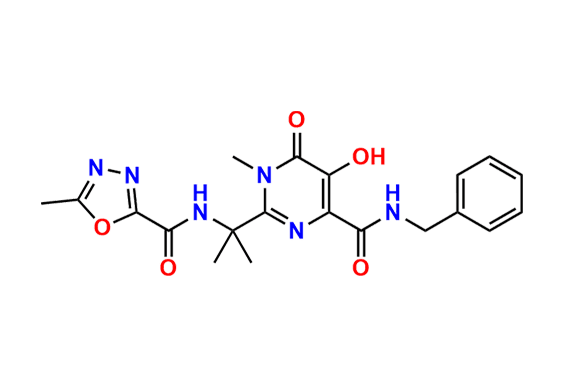
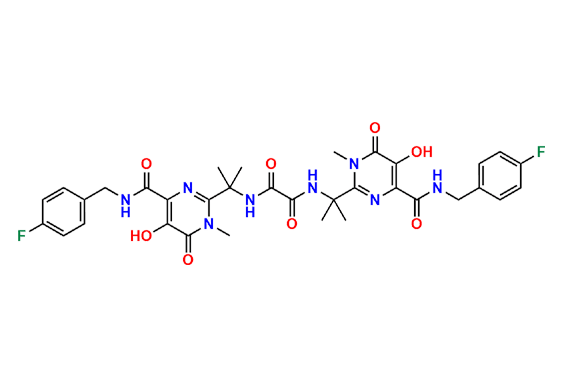
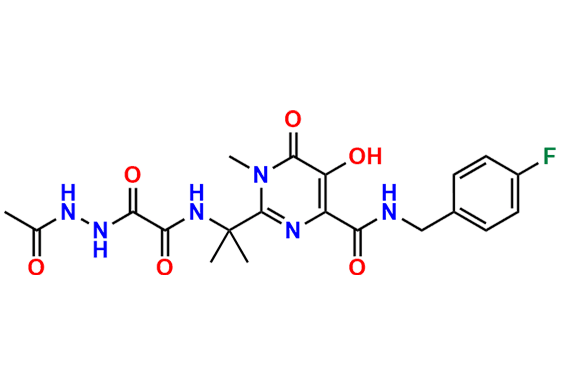
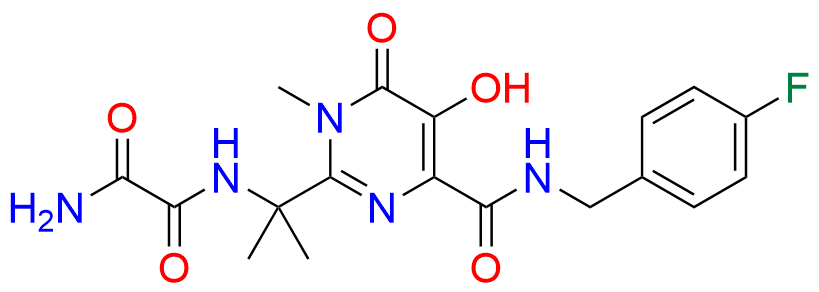
Raltegravir EP Impurity G & F Mixture
| CAT. No. | CP-R6009 |
|---|---|
| CAS. No. | NA |
| Mol. F. | C44H54F2N12O12 |
| Mol. Wt. | 980.98 |
| Stock Status | Custom Synthesis |
- Category: Impurity Standards
- Synonyms: NA
- Chemical Name: Ethyl (E)-N-(2-((2-(4-((4-fluorobenzyl)carbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)amino)-2-oxoacetyl)acetohydrazonate compound with ethyl (Z)-N-(2-((2-(4-((4-fluorobenzyl)carbamoyl)-5-hydroxy-1-methyl-6-oxo-1,6-dihydropyrimidin-2-yl)propan-2-yl)amino)-2-oxoacetyl)acetohydrazonate (1:1)