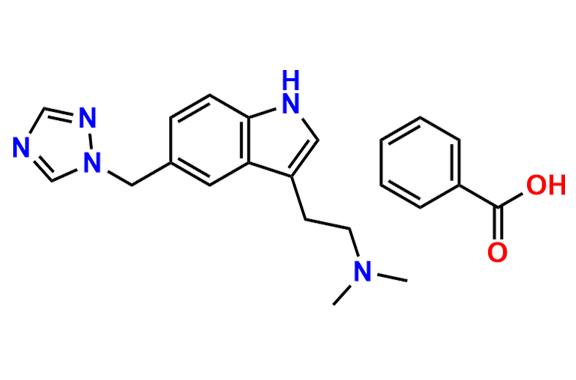
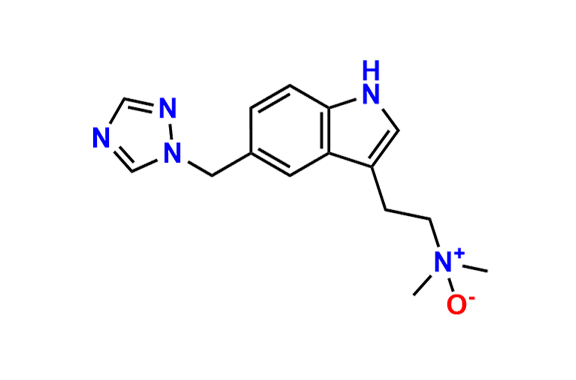
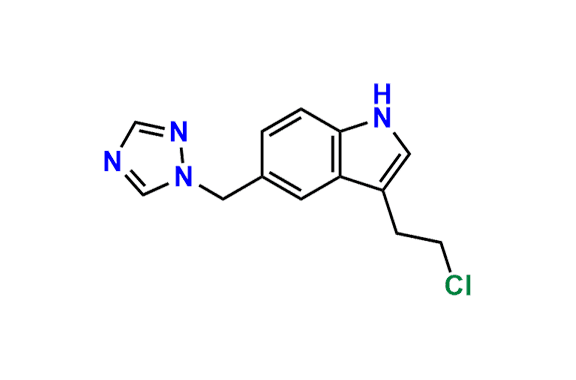
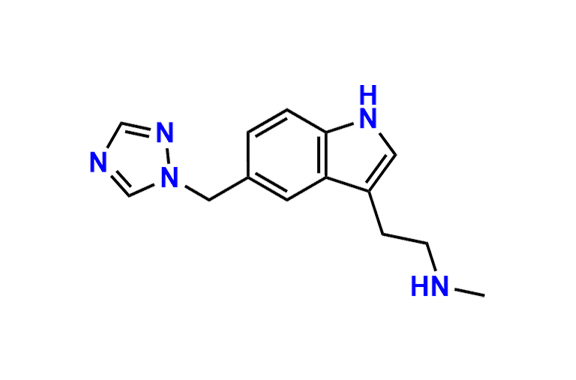
Rizatriptan EP Impurity I
| CAT. No. | CP-R18009 |
|---|---|
| CAS. No. | 144034-84-4 |
| Mol. F. | C14H17N5 |
| Mol. Wt. | 255.33 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: N-Desmethyl Rizatriptan
- Chemical Name: N-Methyl-2-[5-(1H-1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]ethanamine (as per EP)

 VIEW COA
VIEW COA