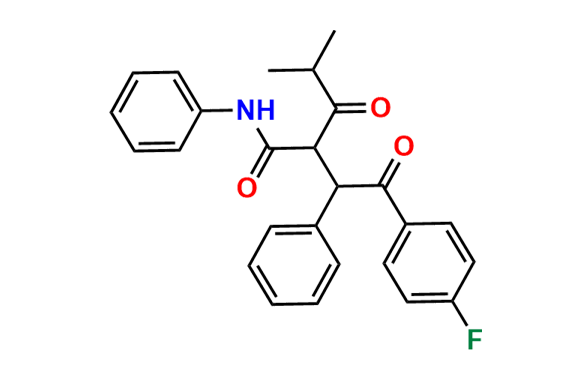
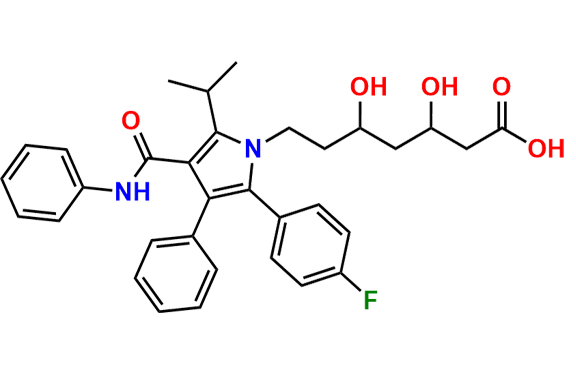
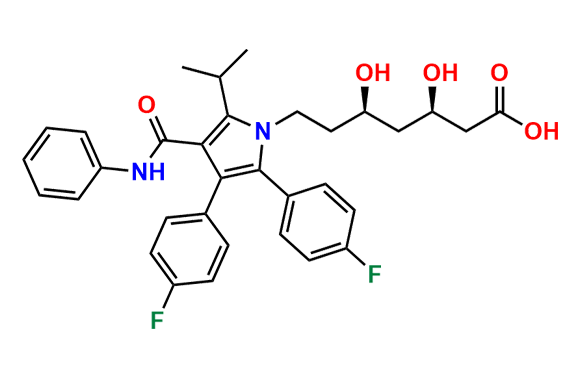
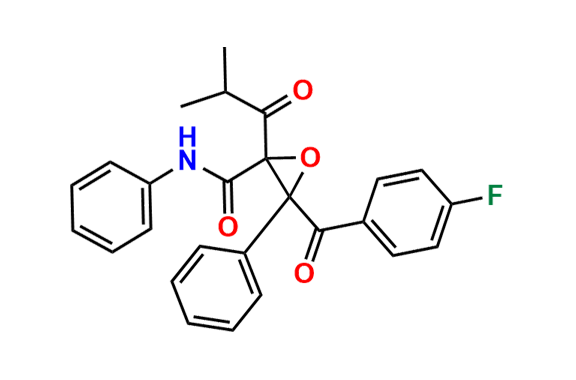
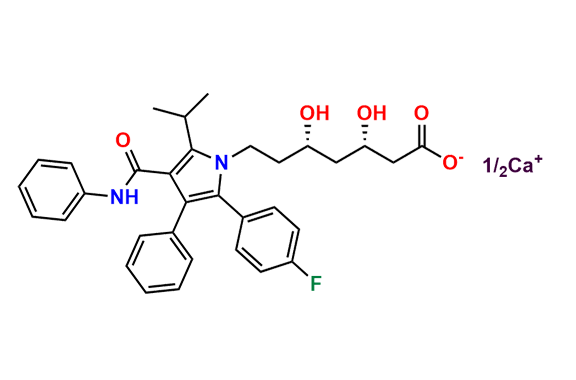
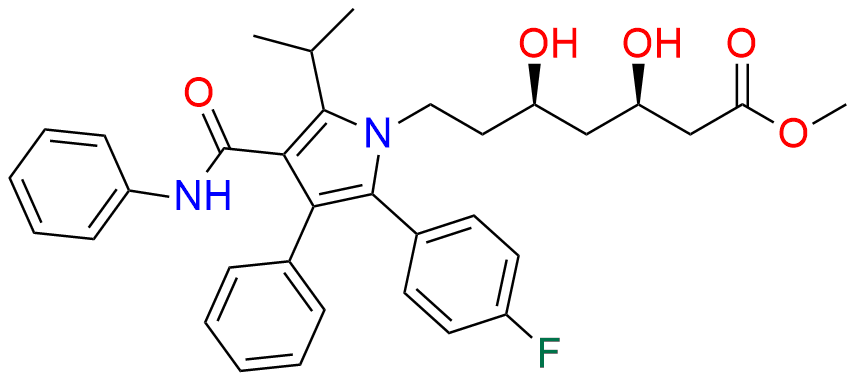
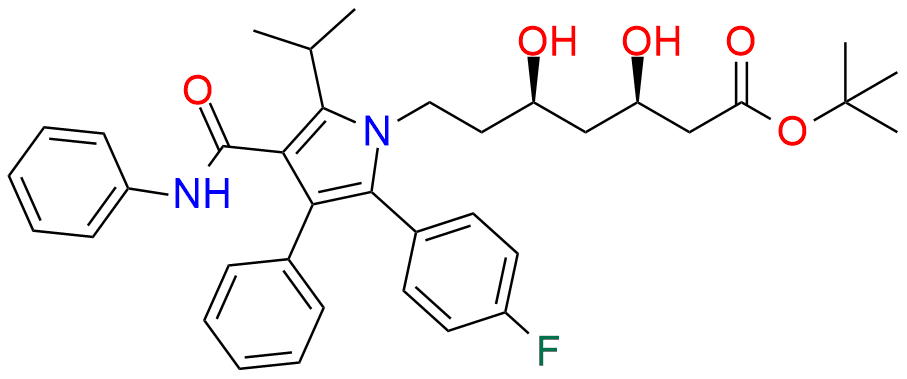
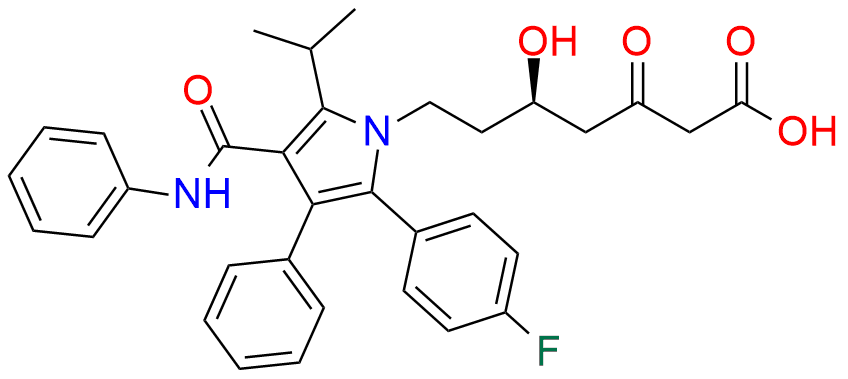
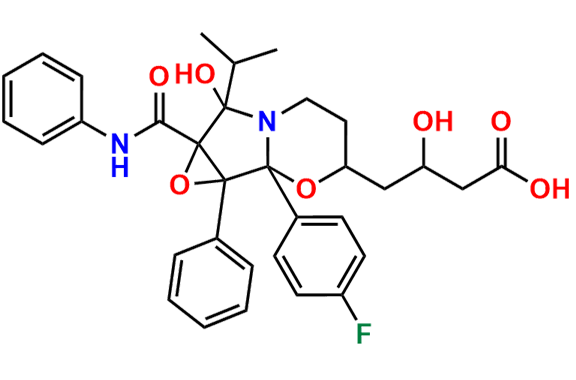
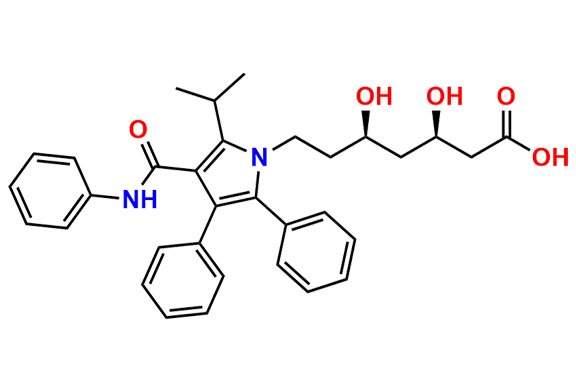
Atorvastatin EP Impurity A
| CAT. No. | CP-A32001 |
|---|---|
| CAS. No. | 433289-84-0 |
| Mol. F. | C33H36N2O5 |
| Mol. Wt. | 540.66 |
| Stock Status | In Stock |
| Rel. Cas No | 1516864-05-3 (Na salt) ; 433289-83-9 (Calcium salt) |
- Category: Impurity Standards
- Synonyms: Desfluoro Atorvastatin ; Atorvastatin USP Related Compound A
- Chemical Name: (3R,5R)-3,5-dihydroxy-7-(2-isopropyl-4,5-diphenyl-3-(phenylcarbamoyl)-1H-pyrrol-1-yl)heptanoic acid

 VIEW COA
VIEW COA