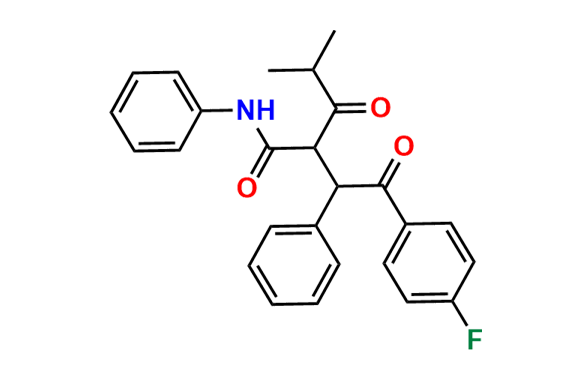
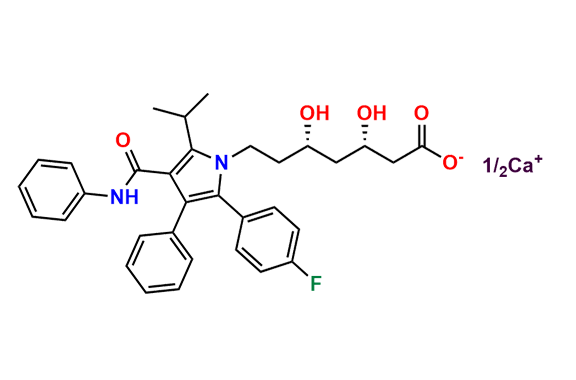
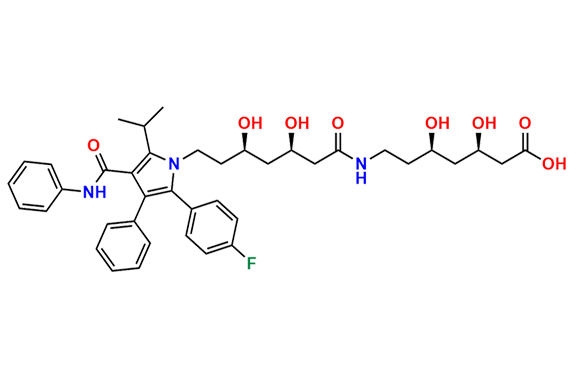
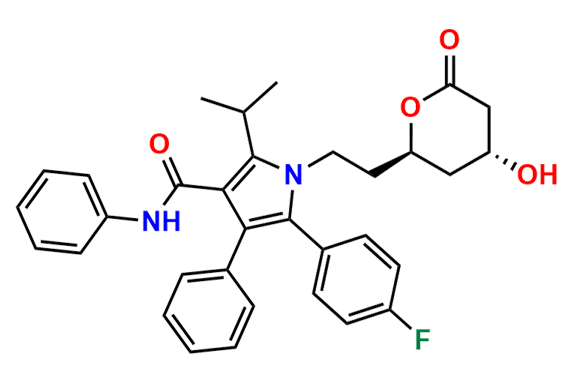
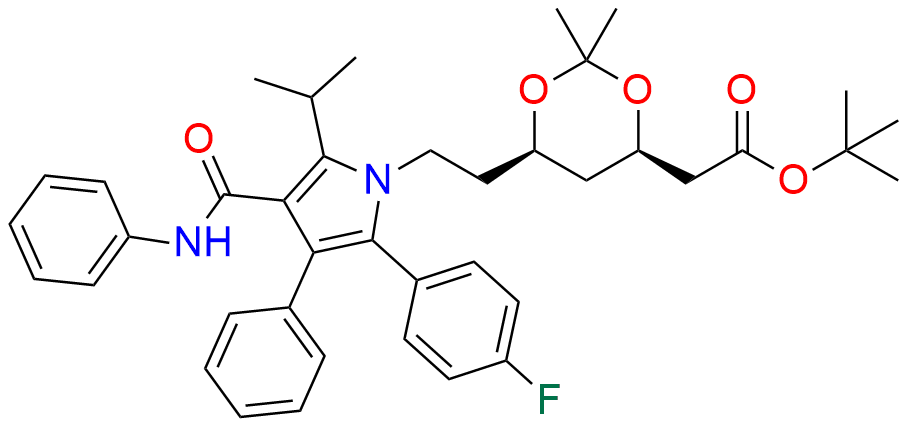
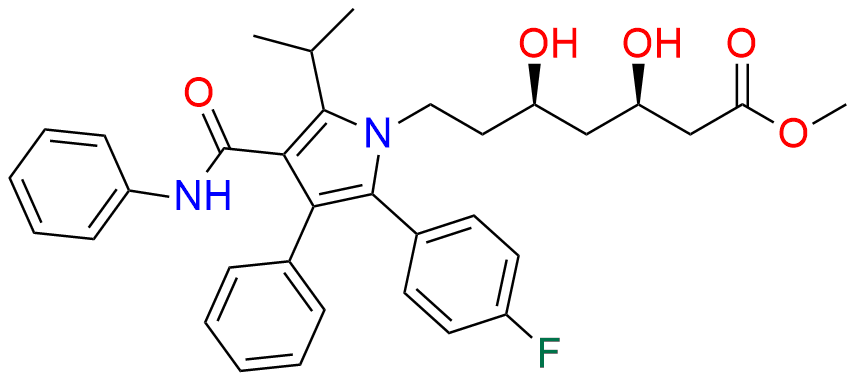
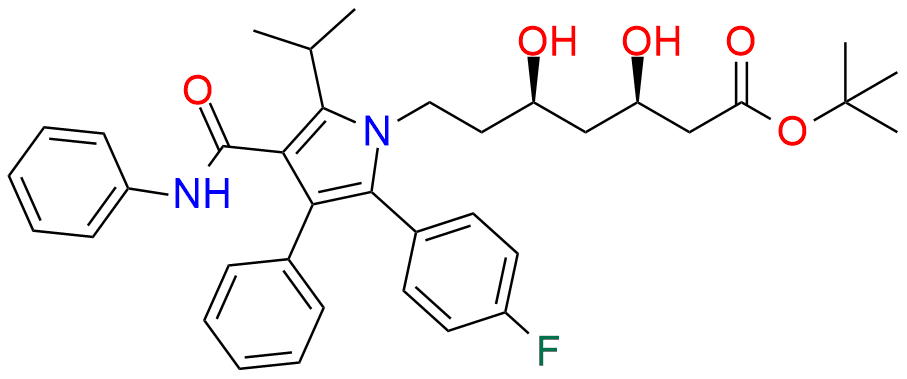
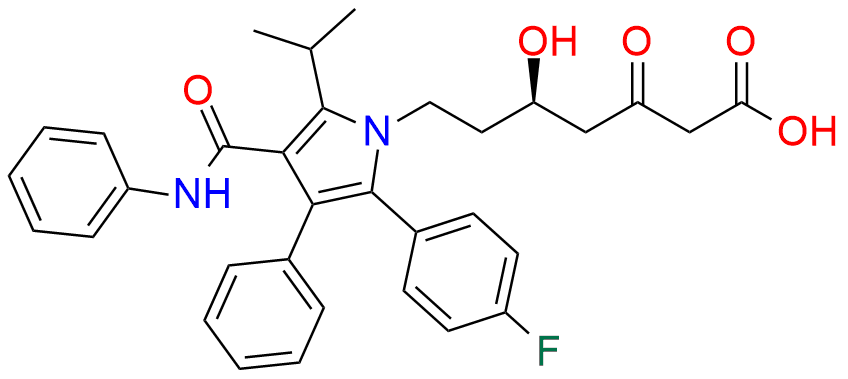
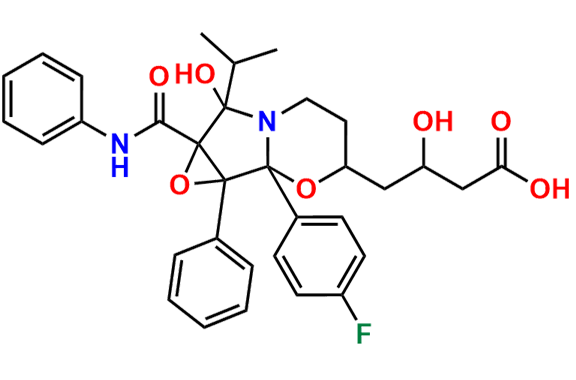
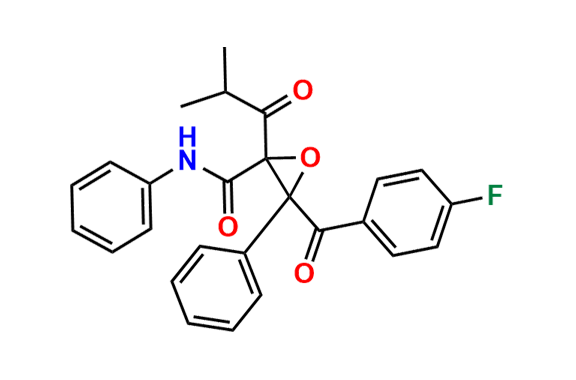
Atorvastatin EP Impurity D
| CAT. No. | CP-A32004 |
|---|---|
| CAS. No. | 148146-51-4 |
| Mol. F. | C26H22FNO4 |
| Mol. Wt. | 431.46 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: Atorvastatin EP Impurity D (D1) ; Atorvastatin USP Related Compound D ; Atorvastatin Diketo Epoxide ; Atorvastatin Degradation Product - ATV-FXA1
- Chemical Name: 3-(4-Fluorobenzoyl)-2-isobutyryl-N,3-diphenyloxirane-2-carboxamide (as per USP)

 VIEW COA
VIEW COA