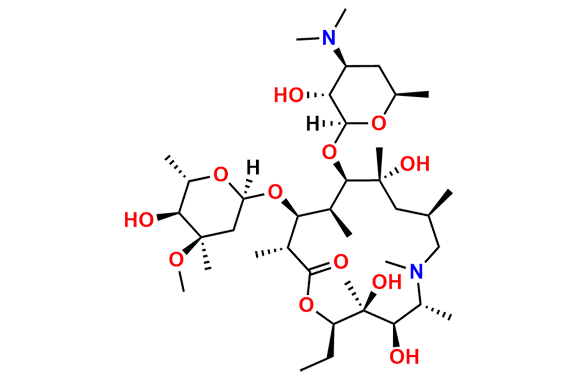
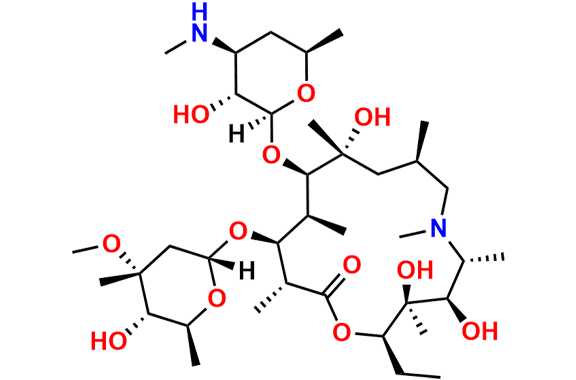
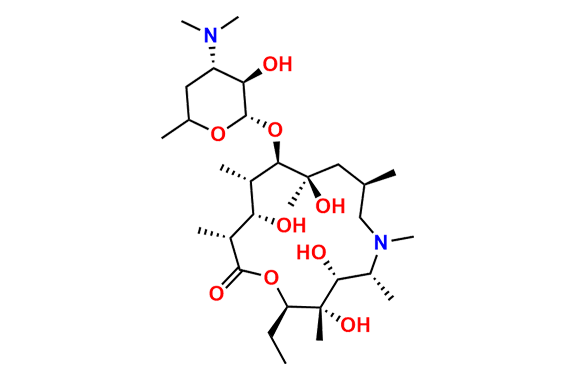
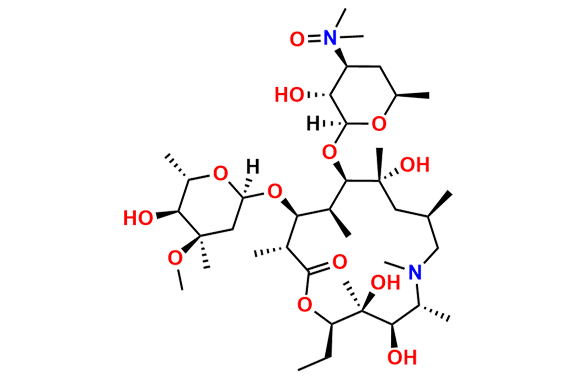
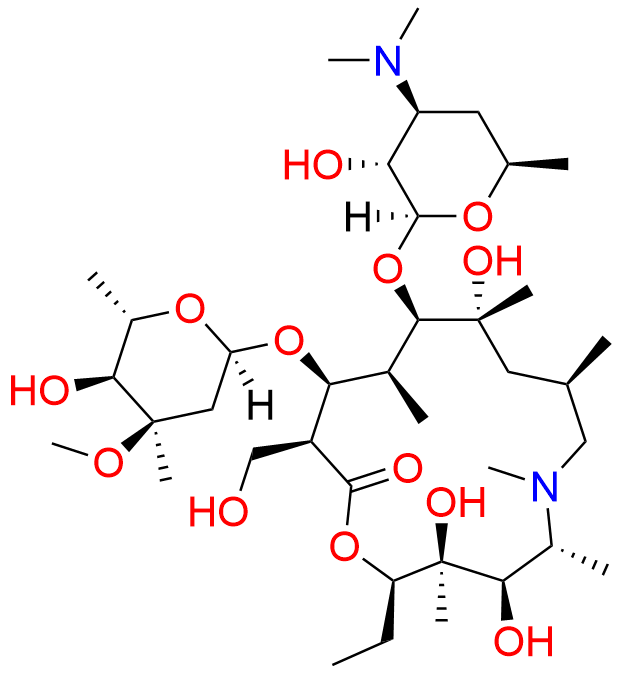
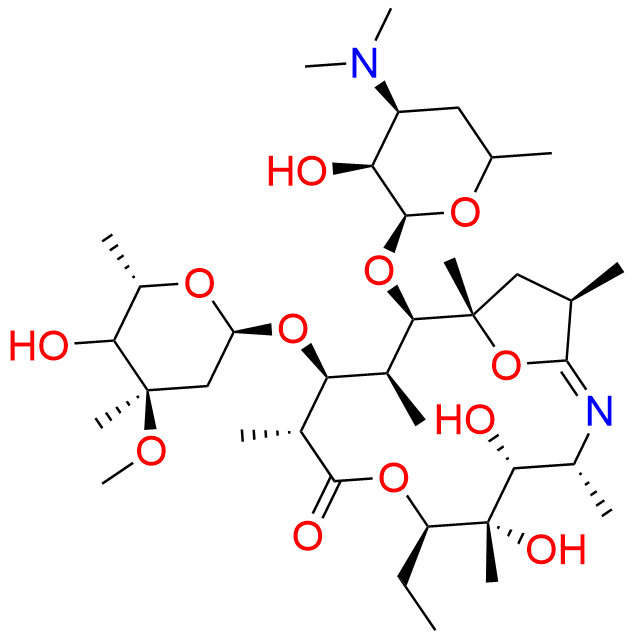
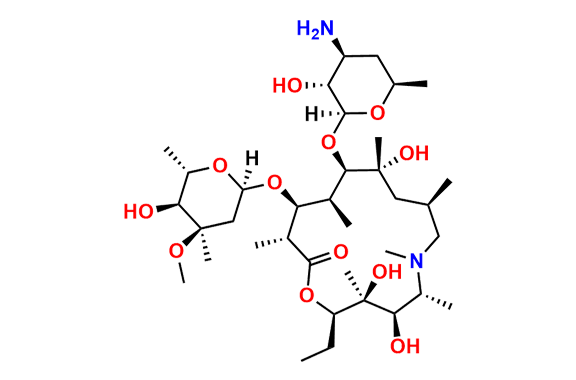
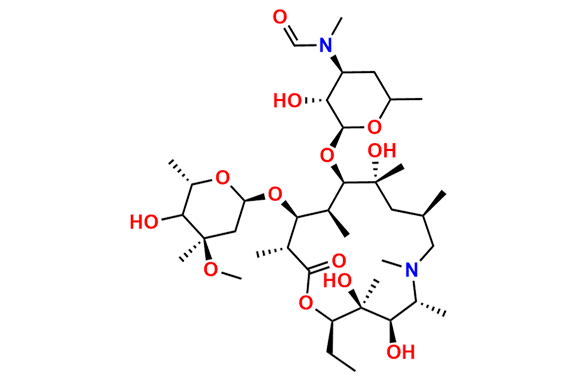
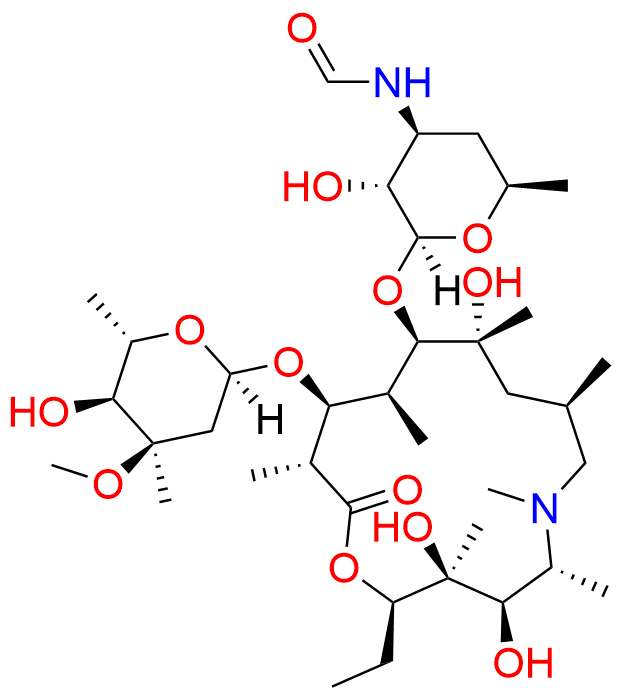
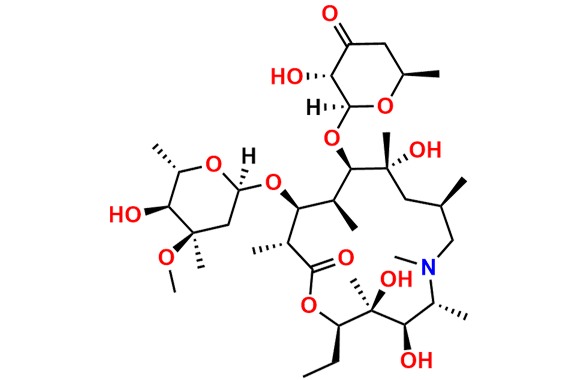
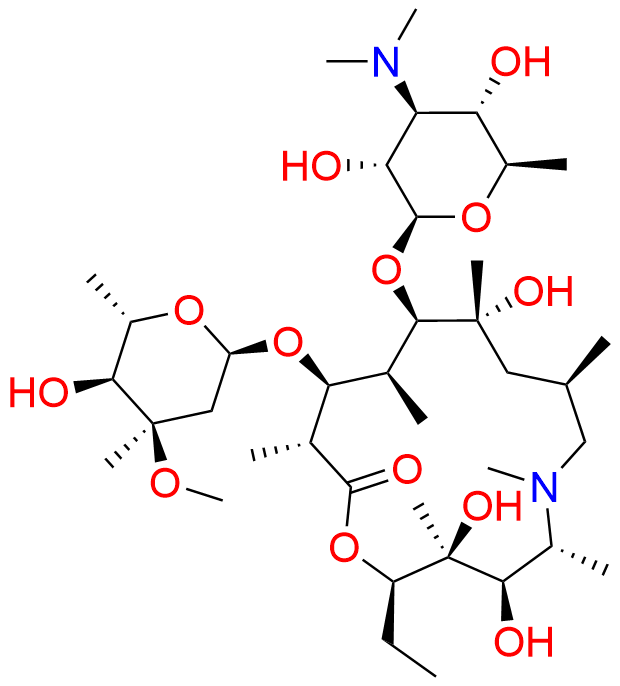
Azithromycin EP Impurity P
| CAT. No. | CP-A37016 |
|---|---|
| CAS. No. | 92594-45-1 |
| Mol. F. | C39H74N2O12 |
| Mol. Wt. | 763.02 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: NA
- Chemical Name: 11-((4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-2,6-diethyl-3,4,10-trihydroxy-13-((5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-3,5,8,10,12,14-hexamethyl-1-oxa-6-azacyclopentadecan-15-one

 VIEW COA
VIEW COA