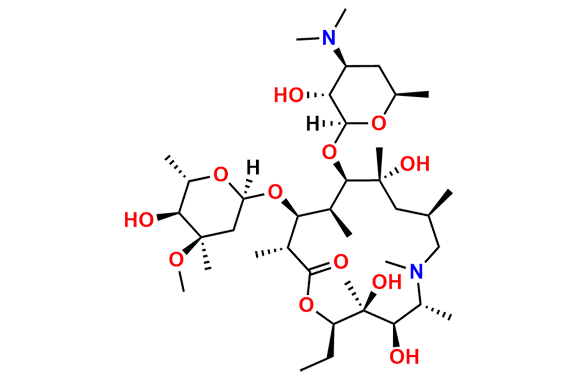
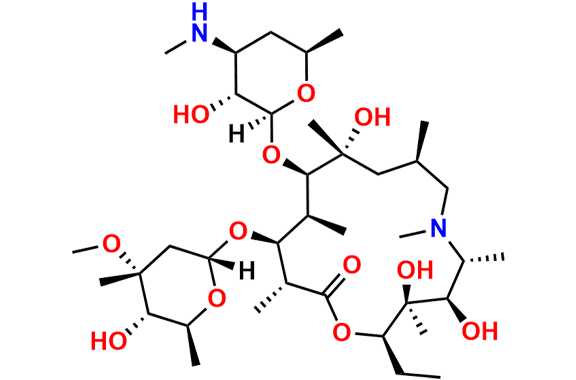
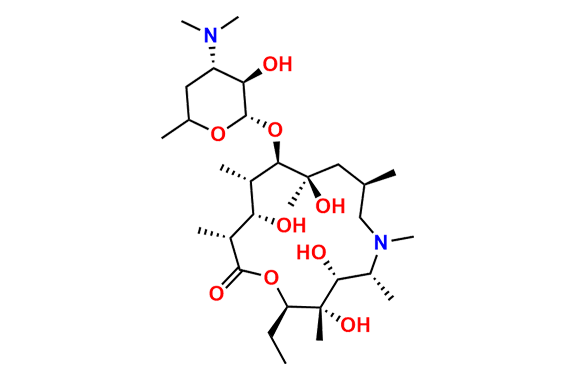
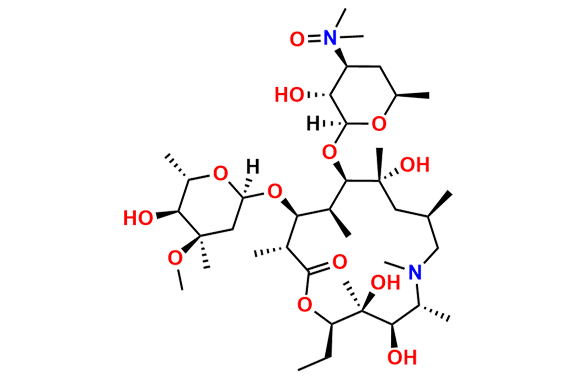
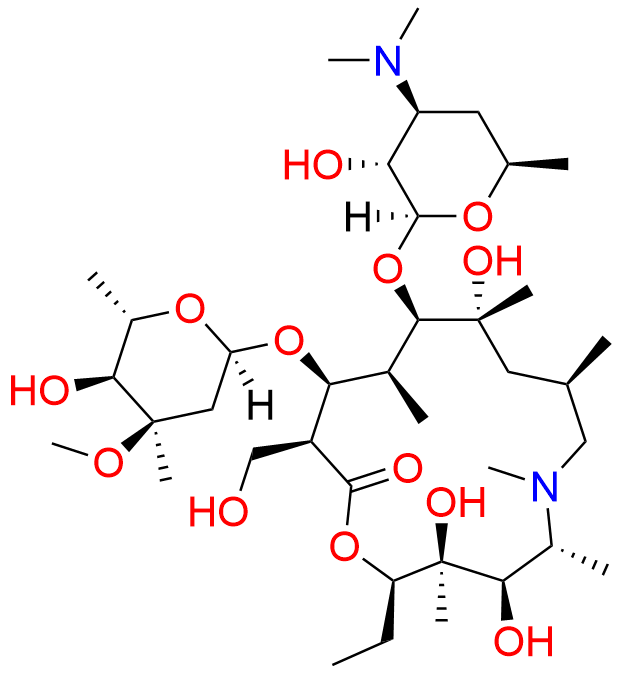
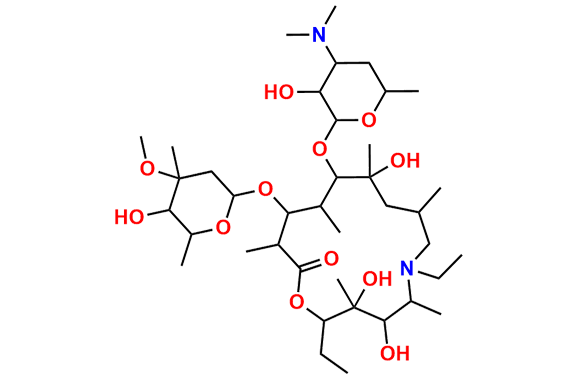
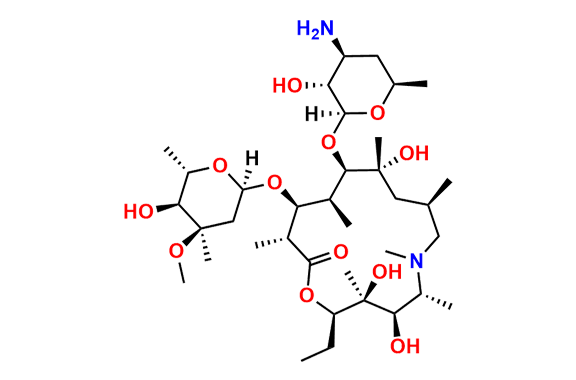
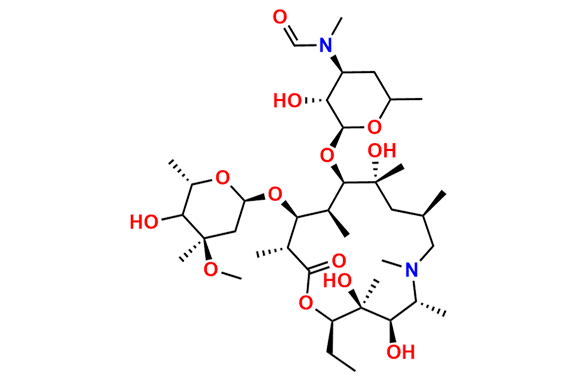
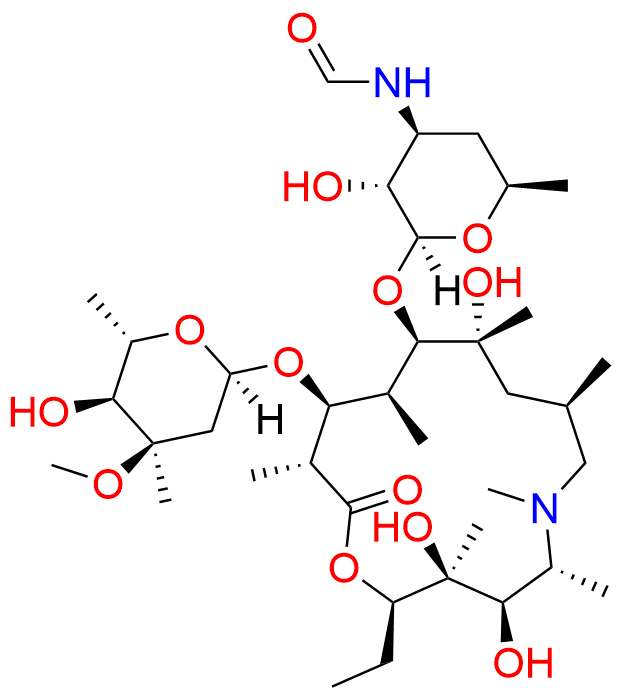
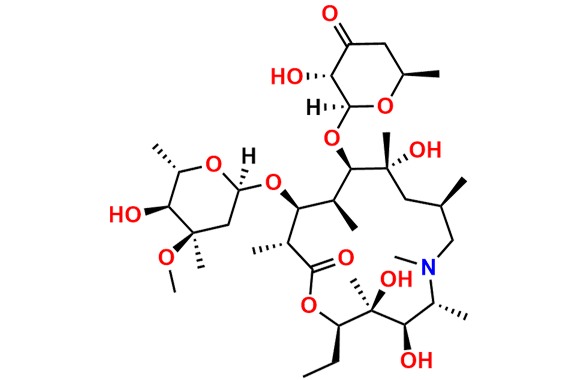
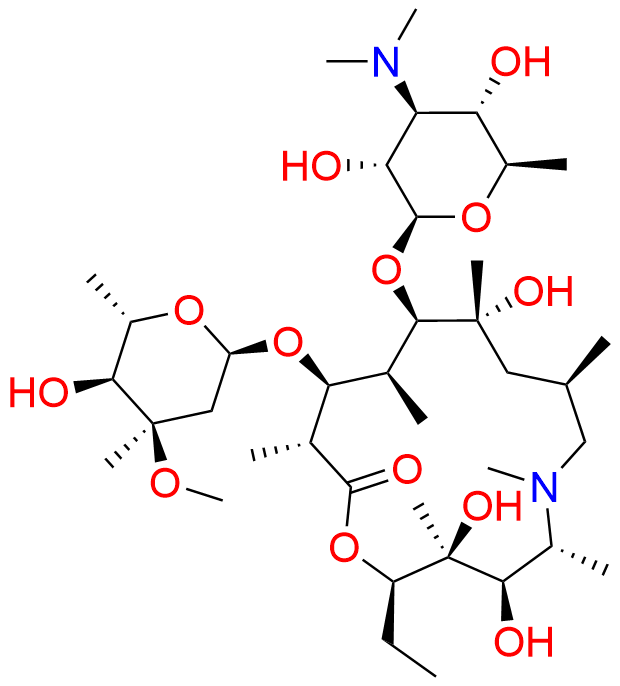
Azithromycin EP Impurity Q
| CAT. No. | CP-A37017 |
|---|---|
| CAS. No. | 2095879-65-3 |
| Mol. F. | C44H75N3O15S |
| Mol. Wt. | 918.1 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: NA
- Chemical Name: 3′-N-[[4-(acetylamino)phenyl]sulfonyl]-3′-(N,N-didemethyl)azithromycin (as per EP)

 VIEW COA
VIEW COA