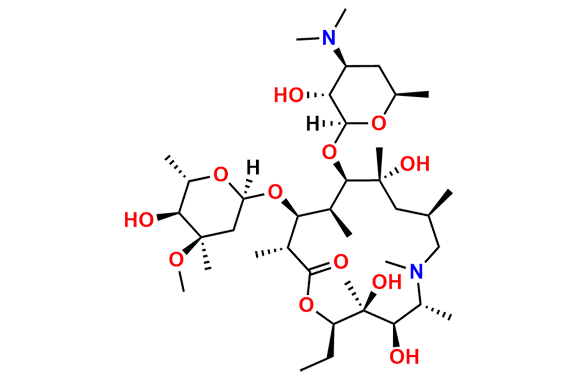
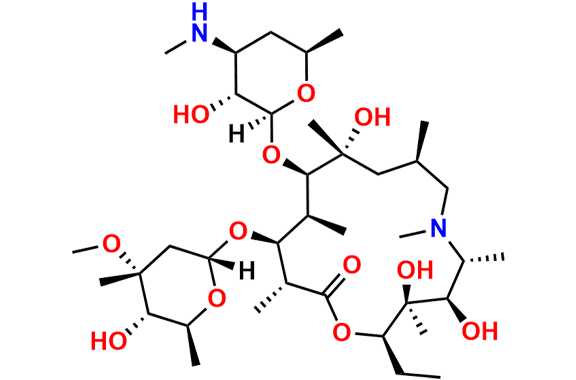
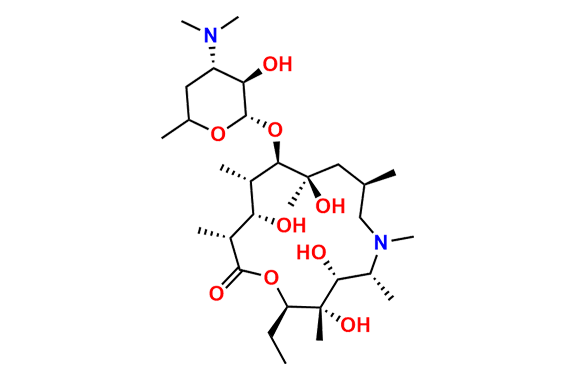
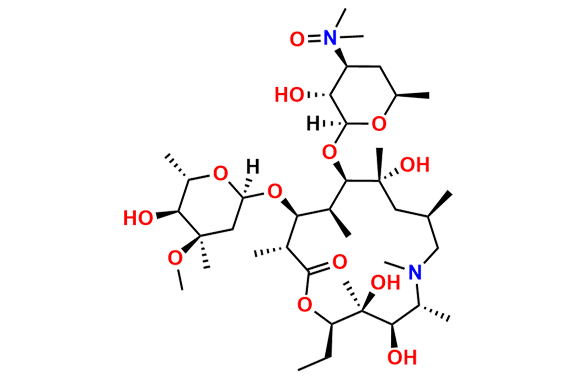
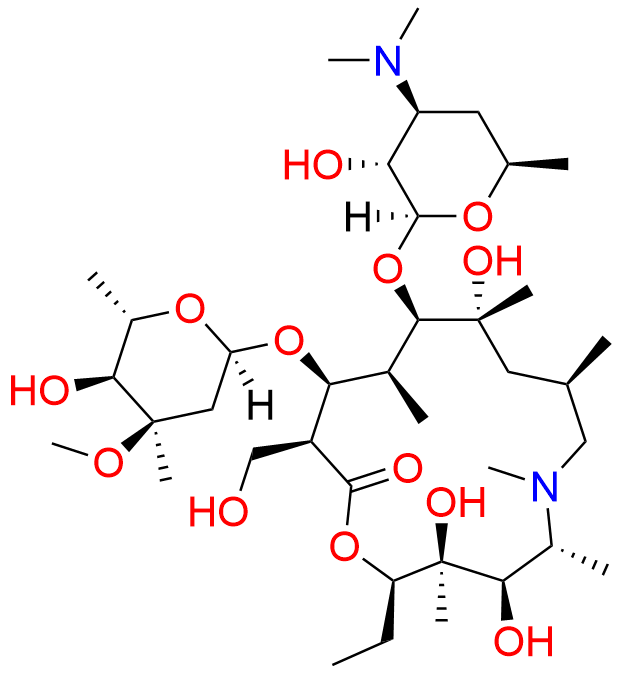
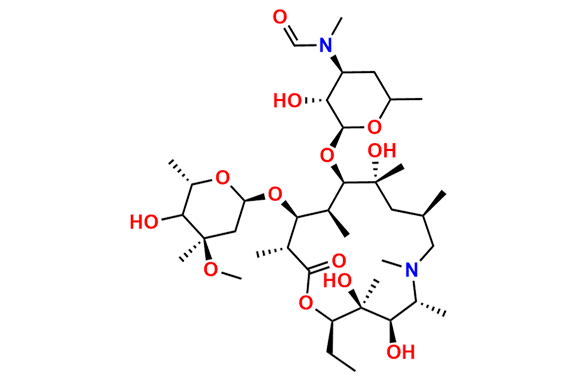
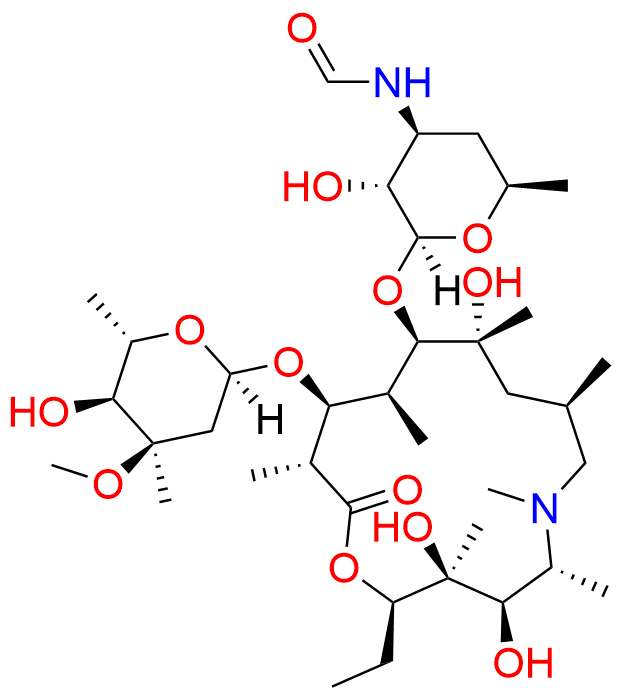
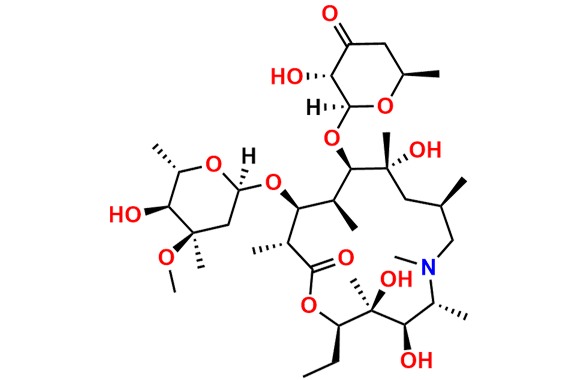
N-Nitroso N-Desmethyl Azithromycin
| CAT. No. | CP-A37022 |
|---|---|
| CAS. No. | NA |
| Mol. F. | C37H69N3O13 |
| Mol. Wt. | 763.96 |
| Stock Status | In Stock |
- Category: Impurity Standards
- Synonyms: N-Nitroso Azithromycin EP Impurity I
- Chemical Name: N-((2S,3R,4S,6R)-2-(((2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-2-ethyl-3,4,10-trihydroxy-13-(((2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)-3,5,6,8,10,12,14-heptamethyl-15-oxo-1-oxa-6-azacyclopentadecan-11-yl)oxy)-3-hydroxy-6-methyltetrahydro-2H-pyran-4-yl)-N-methylnitrous amide
FAQ
It is a nitrosamine impurity that can form during the synthesis, storage, or handling of Azithromycin, commonly used in pharmaceuticals.
It is subject to strict guidelines from regulatory bodies like the FDA and EMA to ensure safe levels in medications.
It forms when Azithromycin interacts with nitrosating agents, especially under conditions involving heat, moisture, or acidity.
Through the use of appropriate materials, optimized storage conditions, pH control, and routine testing.
Techniques like GC-MS (high sensitivity), LC-MS (accurate measurement), and HPLC-MS (flexible analysis) are employed.
Chemicea provides COA, H-NMR, MASS, HPLC, and TGA reports as standard, with additional reports like CNMR, IR, UV, DEPT, Water content, and CHNS available upon request.
Yes, they meet the standards of USFDA, EMA, ANVISA, TGA, PMDA, and other global health agencies.
N-Nitroso Azithromycin EP Impurity I is stable for shipping at room temperature, with specific storage requirements detailed in the COA.
Yes, both standard and customized pack sizes are available to meet specific client needs.
Chemicea provides N-Nitroso Azithromycin EP Impurity I as a reference standard, aiding in method validation, impurity analysis, and regulatory compliance for quality control.

 VIEW COA
VIEW COA